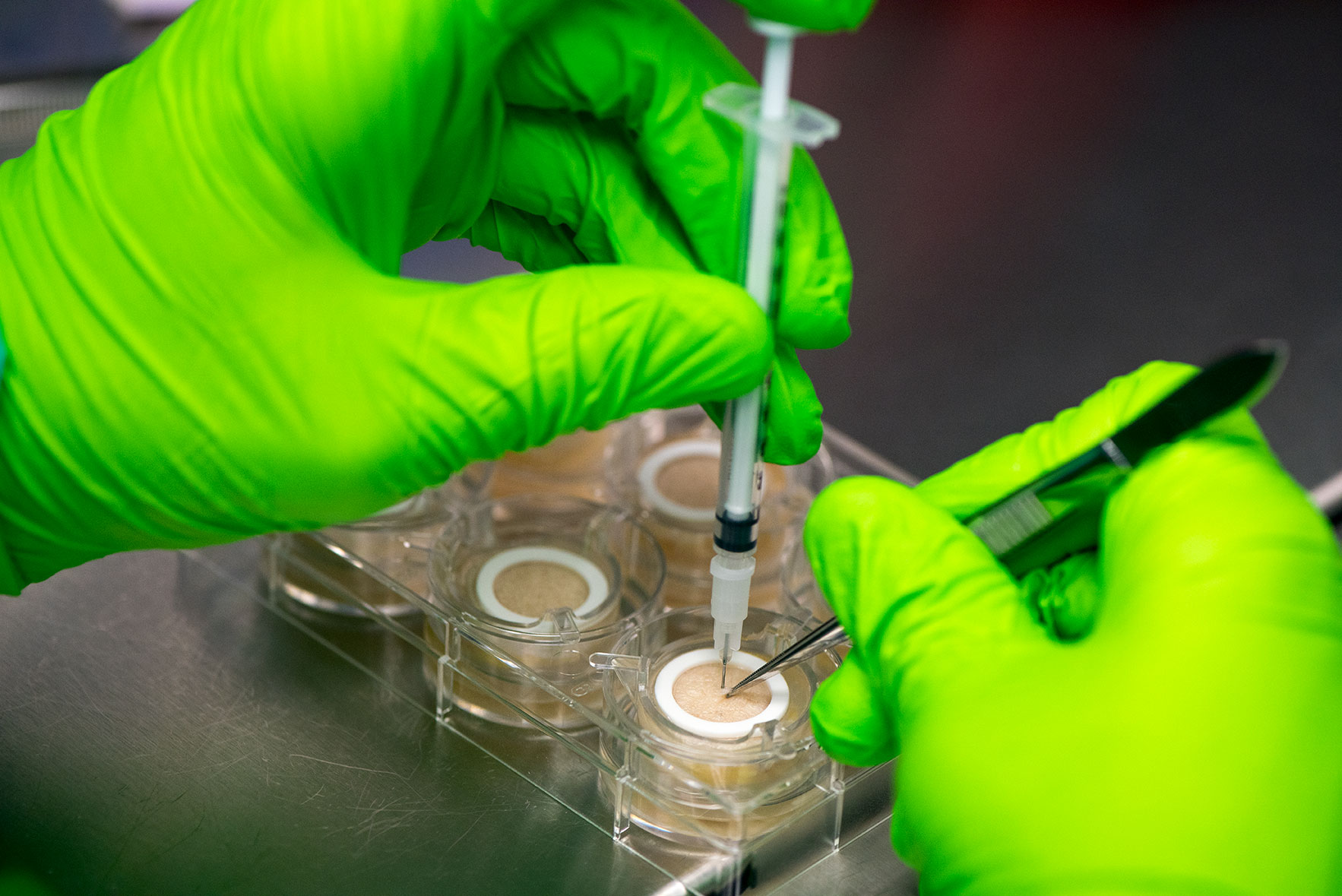
Ex vivo human skin model to predict toxicity and efficacy of subcutaneous drugs
HypoSkin®: a unique ex vivo human skin model for subcutaneous injection testing
Subcutaneous biologics market is an emerging area expanding at an impressive average growth rate of 7% to reach € 161 billion by 2024. To date, there is a lack of relevant in vitro biological models to test the effects of compound injection in the subcutaneous tissue causing a loss of more than €1 billion per drug development (1,2). HypoSkin® is the first and unique, highly predictive and ethical ex vivo human model with normal subcutaneous tissue architecture. This model enables to truly evaluate and optimise a new compound or formulation following subcutaneous injection during the earlier stage of product & drug development (preclinical trials).
A patented technology as a ready-to-use testing kit and as a service
HypoSkin® contains all three layers of the skin: epidermis, dermis and hypodermis also known as subcutaneous or fat tissue. Importantly, Genoskin’s proprietary biological matrix allows keeping normal cell viability and the 3D architecture of subcutaneous tissue model during 7 days. In addition, the model is enough robust and deformable to support injection with a needle of a large volume (125 µL) of liquid formulation in the adipose tissue. This patented technology is currently available as a standardized, ready-to-use testing kit and as a service to adapt to customers’ needs.
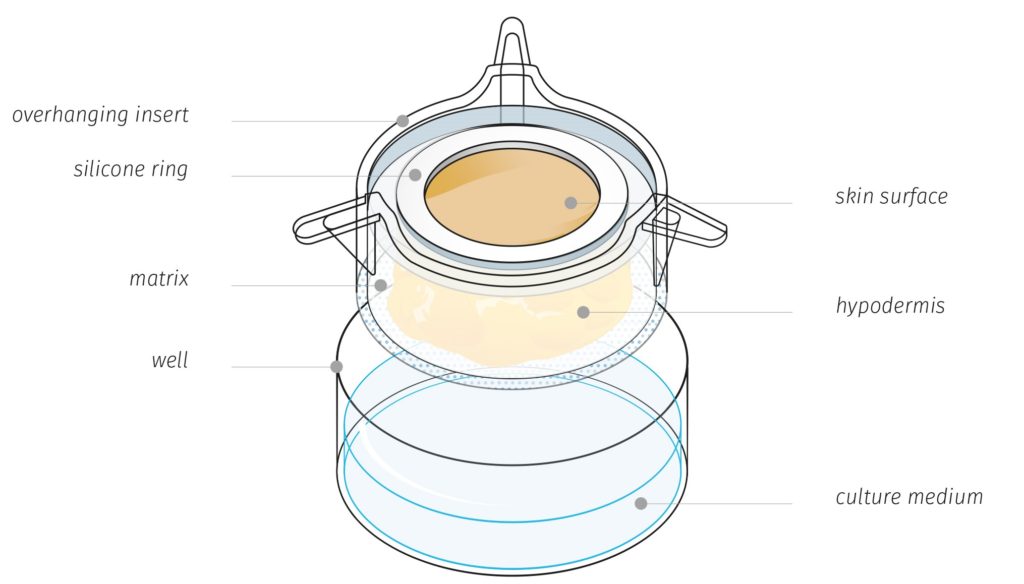
Genoskin’s unique HypoSkin® technology model
HypoSkin® user manual is now available
The document includes a description of HypoSkin® model as well as the different formats that are currently available for subcutaneous injection, topical or systemic administration. It also contains details on how to start upon receipt of the kit, how to administrate a compound, how to cultivate and how to collect skin biopsies following treatment. Finally, a list of approved protocols for analysis is provided. Don’t hesitate to request a copy of our user manual.
Genoskin’s technology backed by EU funding
 This unique breakthrough ex vivo human skin model has received the funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 816289 (SME Instrument Phase 1 program). HypoSkin® technology has also been supported by Genoskin’s customers who have shown interest in this model and have contributed to his validation through collaborative projects.
This unique breakthrough ex vivo human skin model has received the funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 816289 (SME Instrument Phase 1 program). HypoSkin® technology has also been supported by Genoskin’s customers who have shown interest in this model and have contributed to his validation through collaborative projects.
Should you like to learn more about Genoskin’s activities and services, don’t hesitate to contact us.
References
(1) Kola I and Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004, 3 (8):711-5. (2) Arrowsmith J. Trial watch: Phase III and submission failures: 2008-2010. Nat Rev Drug Discov. 2011, 10 (2):87-87.
Comments are closed.