HypoSkin®
Skin model for subcutaneous injections
Research by C. Jardet (Genoskin), E. Pagès (Genoskin), E. Raude (Genoskin), F. Seeliger (AstraZeneca), L. Brandén (AstraZeneca), E. Braun (Genoskin), M. Ingelsten (LAAS-CNRS), P. Descargues (Genoskin)
Evaluation of local inflammatory reactions following subcutaneous injection of a pro-inflammatory cocktail in a fully human ex vivo skin model
Using HypoSkin® ex vivo skin model

Preclinical testing of new subcutaneously injected formulations are generally performed in vivo on animals. However, these models are not recognized as universal and reliable due to the difference in skin and subcutaneous fat tissue when compared to humans leading to low predictability. As a result, there are no accurate biological models to predict toxicity of compounds injected in the subcutaneous tissue. To overcome these issues, we have developed, characterized and validated HypoSkin®, the first and unique, highly predictive and ethical human model to replace animal testing based on the NativeSkin® explant technology.
Normal subcutaneous tissue architecture & presence of macrophages
The integrity of the 3 skin layers was preserved at the macroscopical and histological levels with no sign of necrosis in the epidermis and dermis after 7 days of culture as shown by H&E staining. Cell death was observed starting at day 3 in the hypodermis (white arrows), but with no significant increase after further culture (TUNEL assay). The presence of macrophages (red arrows, anti-CD68 immunostaining) was maintained after 7 days of culture. Data were obtained with an HypoSkin® model 20 mm in diameter with a 10-mm thick adipose tissue.
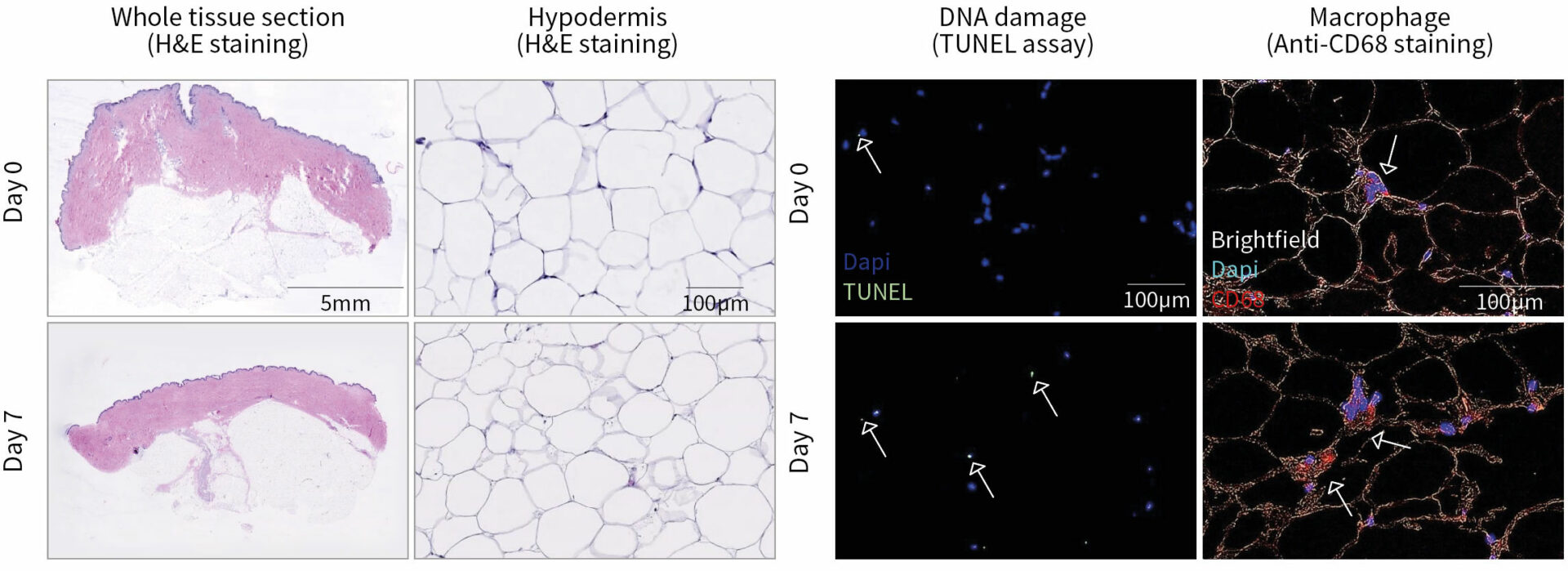
Histological characterization of HypoSkin® model following production & after 7 days of ex vivo culture.
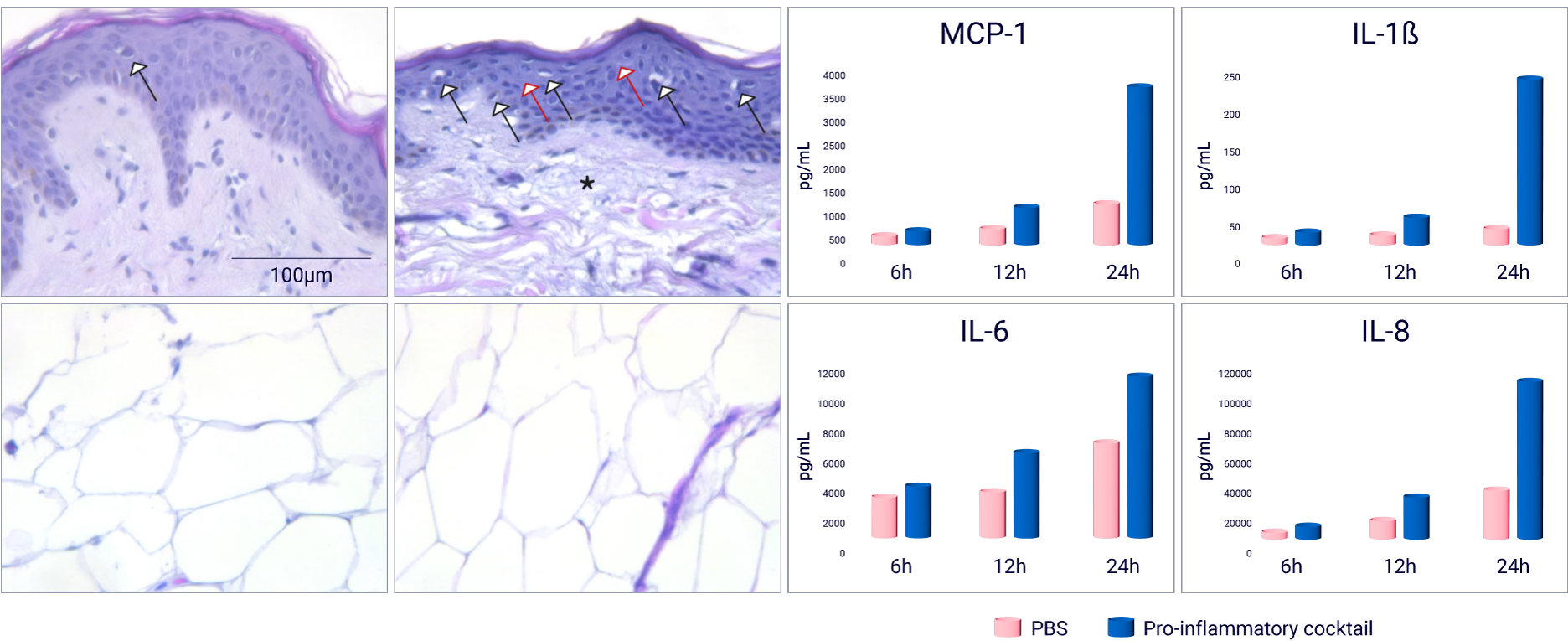
Subcutaneous injection of pro-inflammatory cocktail induces inflammatory reactions
A pro-inflammatory cocktail composed of TNF α and Lipopolysaccharide (LPS) in PBS was subcutaneously injected in HypoSkin® model of 20 mm in diameter and 10 mm thickness. (A) With H&E staining, a normal skin structure was observed at 6 and 12 hours (data not shown) after injection. However, important morphological changes, compared to the PBS injected condition, appeared after 24 hours, including important cell vacuolization (black arrows), spongiotic regions (red arrows) and a partial degradation of collagen fibers (asterisk), while adipose tissue showed normal structure and cell morphology. (B) Injection of the pro-inflammatory cocktail induced a release of cytokines MCP-1, IL-1β, IL-6 and IL-8 after 12 and 24 hours.
Evaluation of local inflammatory reactions following subcutaneous injection of a pro-inflammatory cocktail in HypoSkin® model.
Resident tissue & immune cells produce inflammatory cytokines
The scoring of IL-6 and IL-8 mRNA expression in skin with RNAscope 6 hours following subcutaneous injection of a pro-inflammatory cocktail showed a strong signal from leucocytes, fibroblasts, adipocytes and endothelial cells.
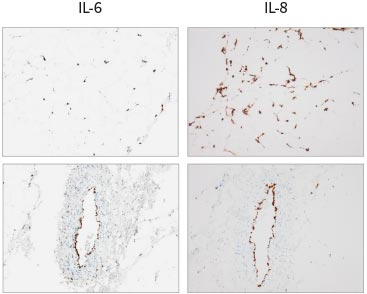
Expression of cytokine mRNA in HypoSkin® model after treatment with a pro-inflammatory cocktail.