Skin Models
NativeSkin®, an immunocompetent human skin model to study antigen uptake and processing by Langerhans cells
Research by E. Pagès1, L. Mondoulet3, E. Bonnefont1, E. Braun1, V. Dhelft2, C. Dupont3, H. Sampson2, P. Descargues1
1Genoskin, 2DBV Technologies, 3Hôpital Necker, Université Paris-Descartes
Scientific Context
Epicutaneous immunotherapy (EPIT) using Viaskin® patches has been shown to induce tolerance in sensitized mice (Dioszeghy, Clin Exp Allergy 2014). The safety and optimal dosing of Viaskin® Peanut for peanut-allergic patients have been explored in earlier Phase 1 and 2 clinical trials, with a Phase 3 safety and efficacy trial successfully completed. In preliminary studies with sensitized mice, DBV Technologies demonstrated that allergens delivered by Viaskin® are mainly taken up by Langerhans cells (LCs) and transported to regional lymph nodes. This study aims to investigate whether similar allergen uptake occurs in an ex vivo human skin model.
Viaskin® patches does not induce major alterations to NativeSkin®‘s structure
The experiment was performed using skin from two donors, with each condition tested in triplicate. Viaskin® patches were applied on the skin after the production of NativeSkin® models.
For each condition, representative images of the models and cross-sections of the epidermis and dermis were captured. Results for donor 2 are shown and were consistent with those from donor 1. In macroscopical images, allergen solubilization due to perspiration can be observed.
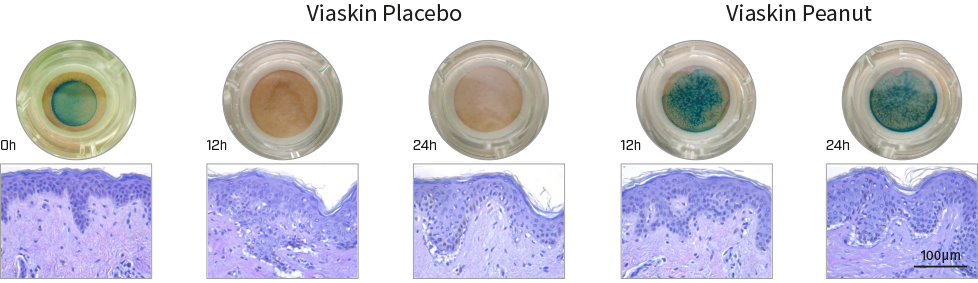
Macroscopical and histological evaluation of tissue integrity during treatment.
Please fill in the form below to access the full poster.
After submitting this form, you will receive an email with the link to access our poster.
Comments are closed.






