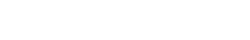ImmunoSafe: ISR platform®
Advancing towards the prediction of clinical results
ImmunoSafe: ISR platform®
Advancing towards the prediction of clinical results
Immunotoxicity assessment with ImmunoSafe: ISR Platform®

Utilizing the foundation of the ISR Platform®, the newly introduced ImmunoSafe: ISR Platform offers a specialized approach to non-clinical predict of immune-related adverse reactions. It integrates ex vivo cytokine analysis, cutting-edge computational analysis, and in-depth literature mining to deduce statistically significant active biological pathways that are activated at the injection site.
Local immunotoxicity testing to de-risk development of human therapeutics
The ImmunoSafe: ISR platform® is an innovative service engineered to reduce the risks associated with the injection of therapeutic agents and formulations.
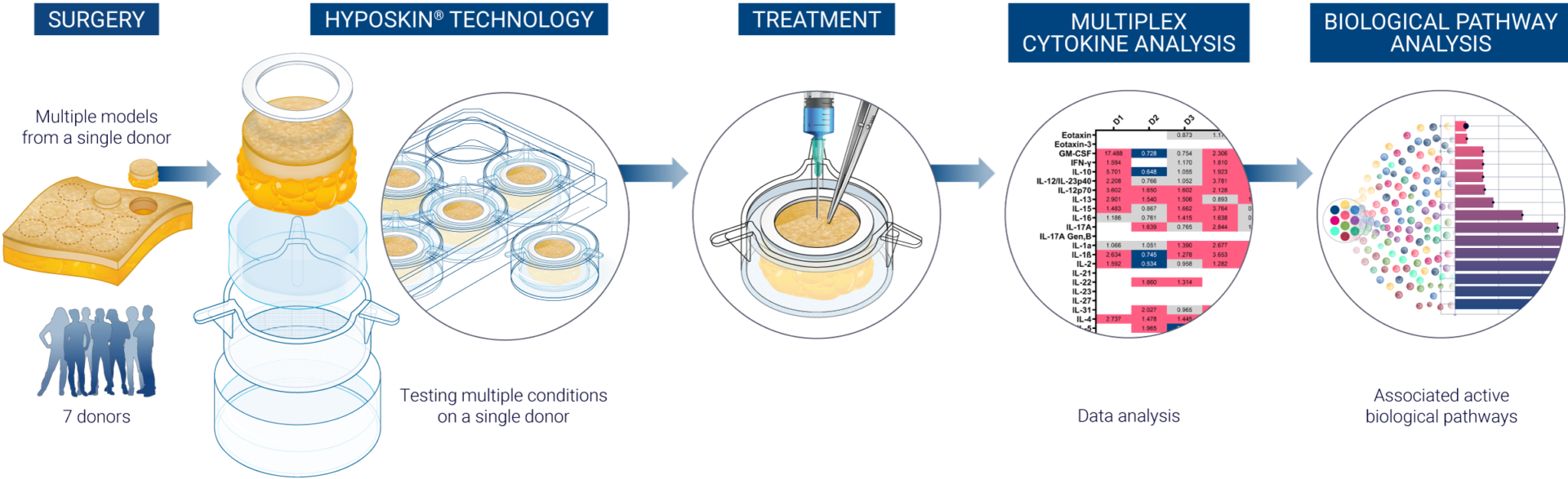
This proprietary state-of-the-art platform provides biotechnology and pharmaceutical companies with the capability to examine how their therapeutic compounds interact with human immune cells in an ex vivo skin, using HypoSkin®. Leveraging advanced computational safety analysis, we ensure a precise evaluation of these interactions. To achieve a comprehensive and diversified understanding of the immune response to various treatments, we utilize a cohort of seven donors.
The data collected from these immune profiles is analysed using AUDACY, our bioinformatics-based analytics solution. The goal of this analysis is to derive significant insights regarding potential clinical outcomes. AUDACY pinpoints active biologically pathways triggered at the injection site that show statistical significance when we administer a compound to our ex vivo skin models.
Bioinformatics-based analysis of multiplex cytokine assays: AUDACY
After subcutaneous or intradermal treatment of models at Day 1, culture medium are sampled to perform multiplex cytokine analysis.
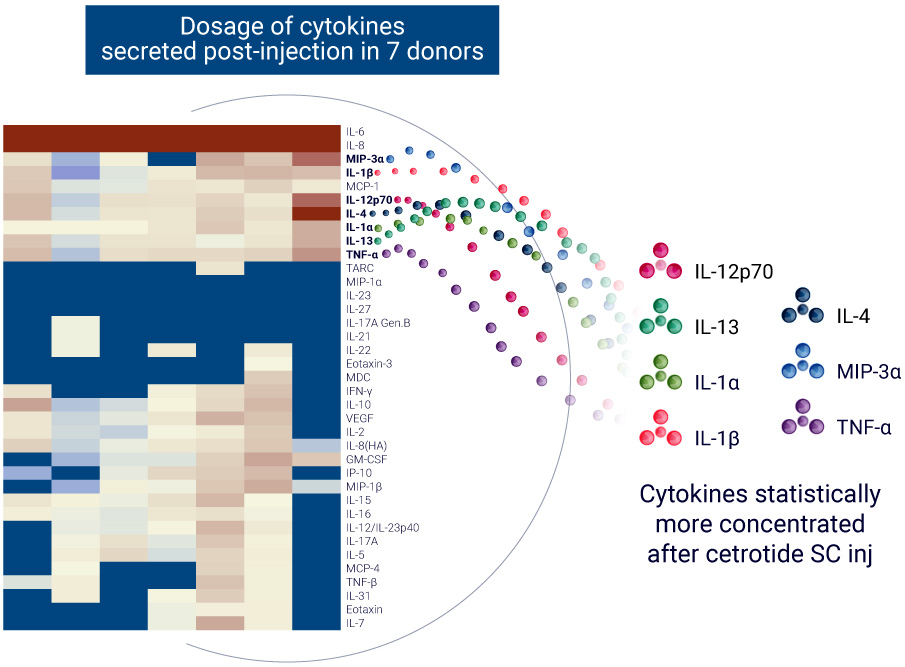
The ImmunoSafe: ISR Platform® uses models from 7 donors with 3 replicates to ensure statistically significant results of cytokine expression. This allows for more accurate characterization of the activated biological pathways implicated in the local immune response.
The exponential increase in biological data complexity has reached a point where even highly skilled experts find data analysis incredibly challenging. With this in mind, Genoskin created AUDACY (Automated Data Analysis of Cytokines), a proprietary bioinformatics-based analytical solution.
AUDACY is designed to:
- Manage large datasets and provide results quickly,
- Minimize the introduction of human error into the analysis,
- Enable novel forms of analysis.
Biological pathways associated with cytokine release in response to Cetrorelix treatment
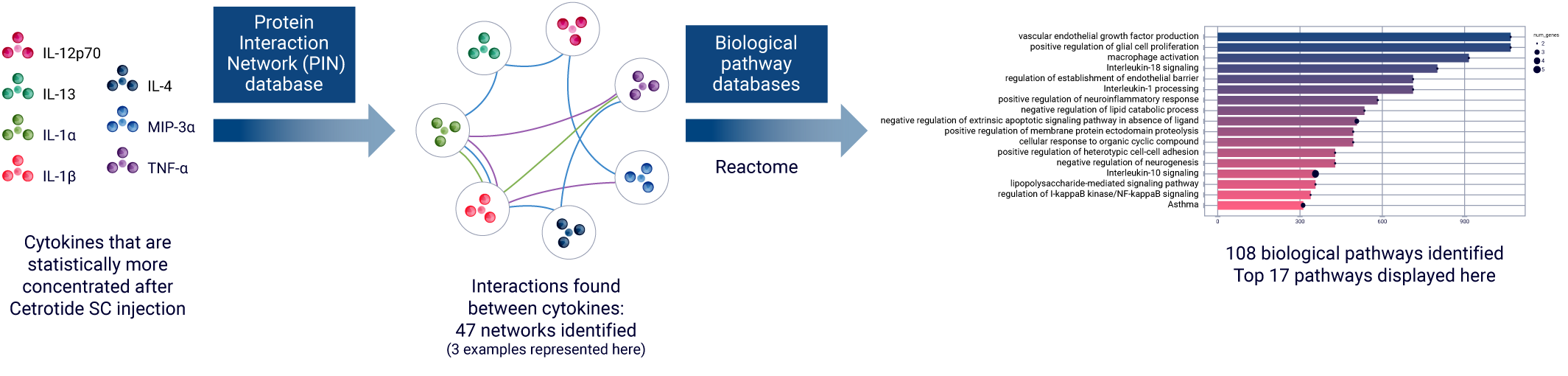
AUDACY uses data from publicly-available, peer-reviewed online databases to gather data from various species. As AUDACY incorporates unique ex vivo human data, we can selectively extract human-related information, such as human gene and protein names.
Based on the list of cytokines and chemokines derived from the statistical analysis, AUDACY uses the Protein Interaction Network (PIN) databases to understand their interconnections. The second step entails examining biological pathway databases (such as Reactome) to gain insights into the specific mechanisms that have been activated. Ultimately, the data enable the inference of clinically-related manifestations.
Frequently asked questions
Who is the ImmunoSafe: ISR Platform® for?
For small molecule therapeutics that trigger local inflammation and cytotoxic tissue damage, our Local Tolerance: ISR Platform® is better suited to address these specific toxicity concerns. Both platforms provide critical data to streamline development and improve drug safety profiles.