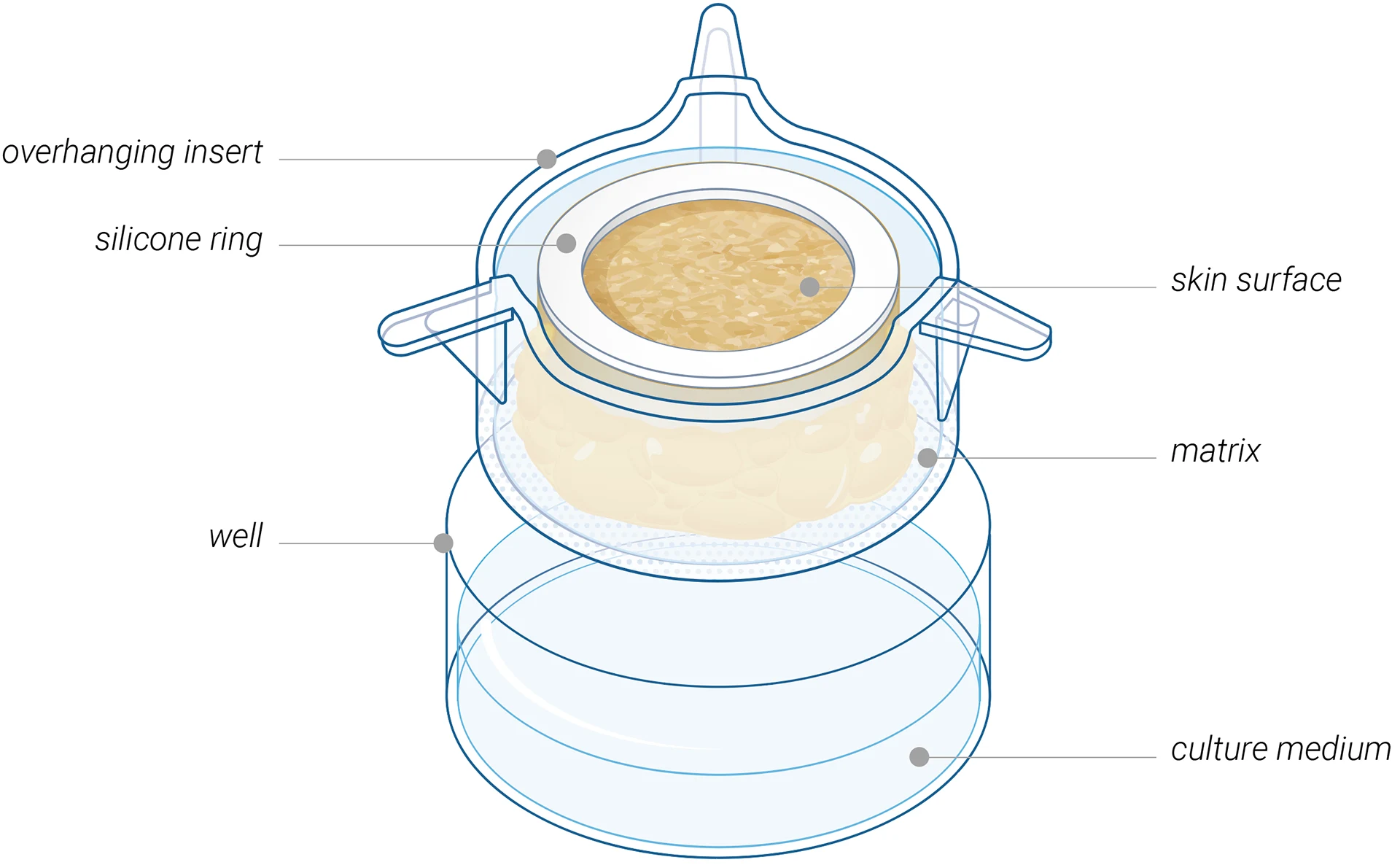
Hyposkin®
Biostabilized ex vivo human skin model
A versatile ex vivo human skin model
designed for testing injectable therapeutics

Biostabilized & functional
- Viable and functional for 7+ days
- Immunocompetent
- Contains 14 immune cell types naturally presented in human skin
Multiple administration routes
- Subcutaneous & intradermal injections
- Topical & transdermal applications
- Systemic-like administration
HypoSkin® is also suitable for medical devices testing.
Biological & immunological validation
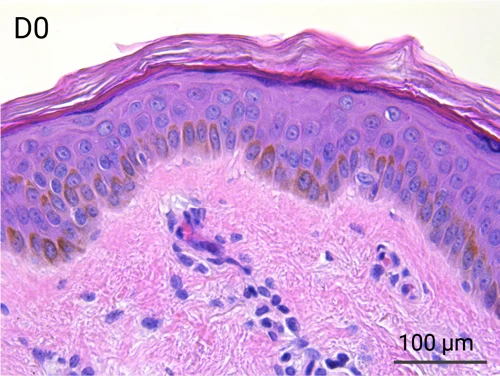
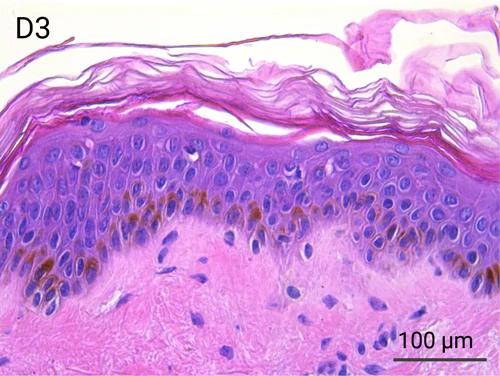
Human skin structure and its resident immune cells are maintained for 7 days

Since we use real human skin to generate HypoSkin®models, they have the same structure and composition as in vivo human skin. Hematoxylin & Eosin staining confirms the preservation of tissue and cellular structures across all three skin layers (epidermis, dermis, and hypodermis). Visual examination reveals a mature stratum corneum, a dermal-epidermal junction mirroring real human skin, and intact basal layers and rete ridges. Notably, the images also demonstrate the sustained integrity of HypoSkin® for a duration of up to 7 days.
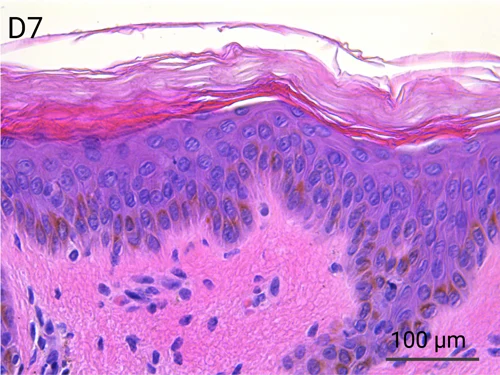
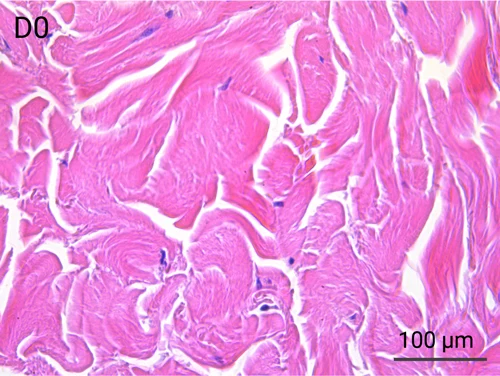
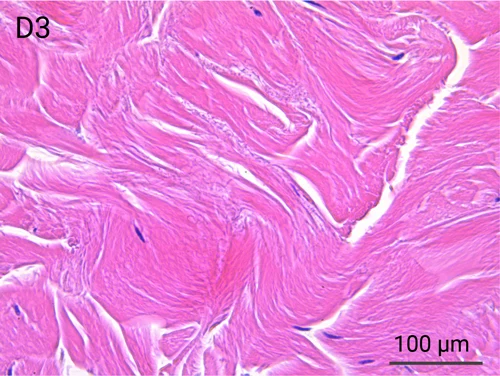
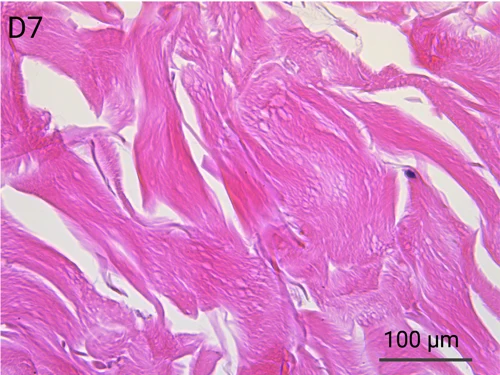
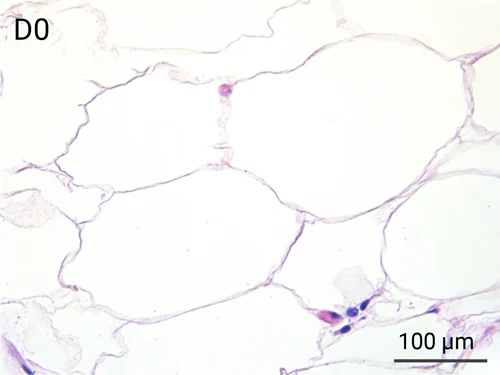
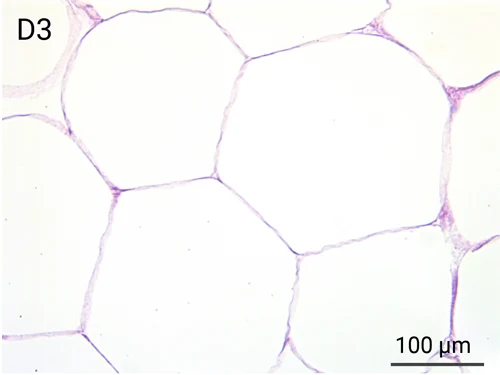
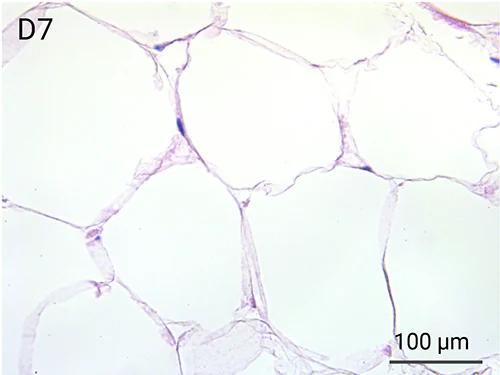
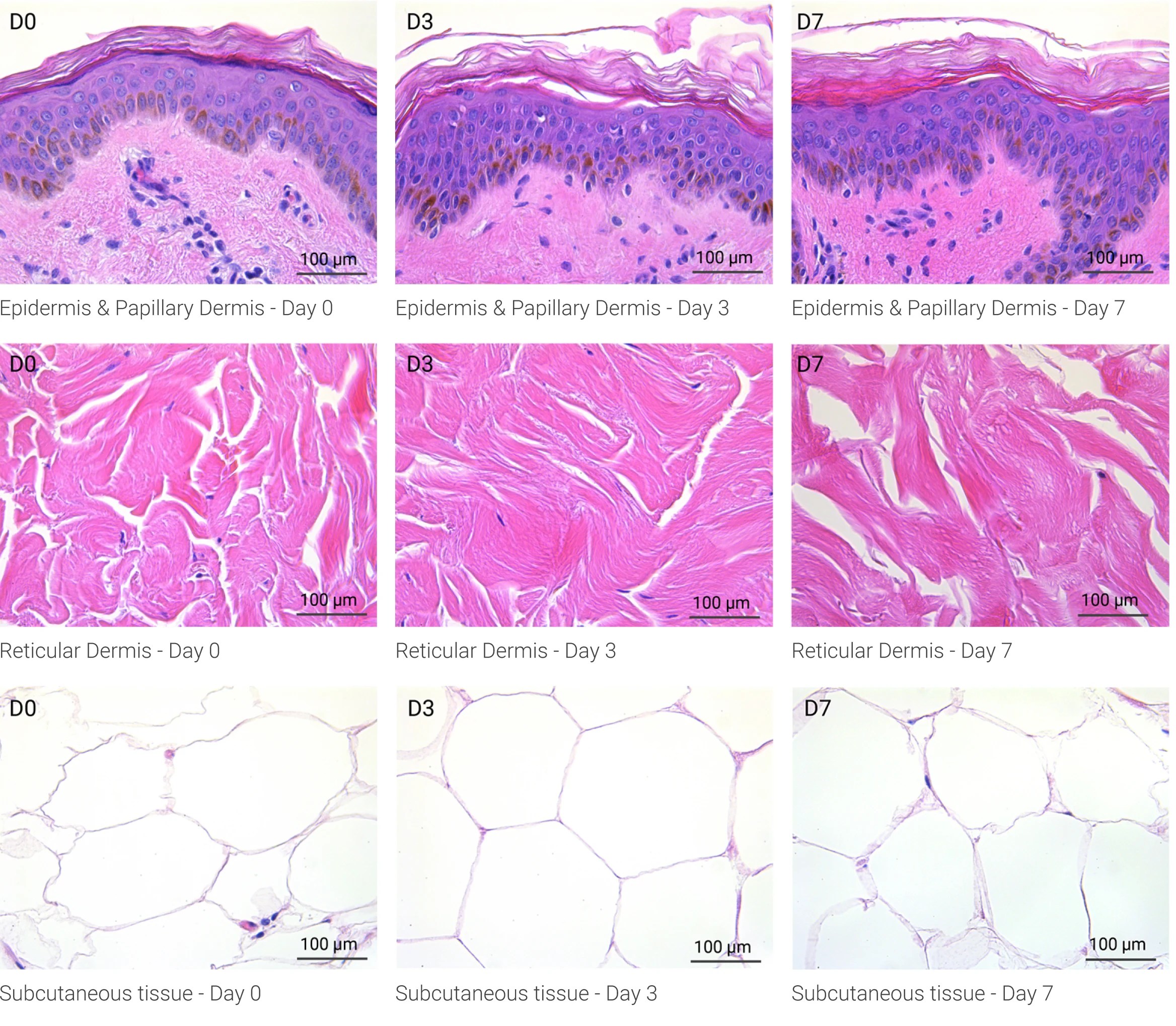
Presence of skin-resident immune cells at 7 days
Genoskin’s proprietary technology enables the maintenance of all skin-resident immune cells for over a week during experimentation.
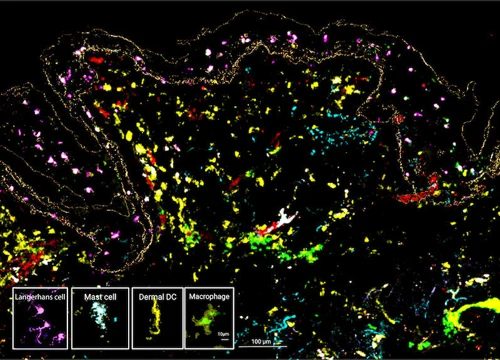
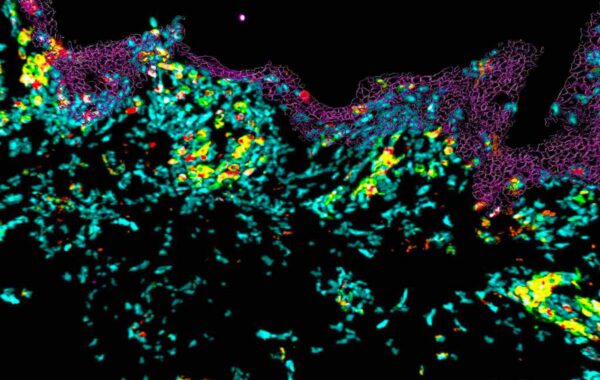
The MANTIS® spatial biology platform enables visualization of immune cell locations within the skin layers and their relative proximity to other cells in HypoSkin® models. The Myeloid Panel is shown here where localization of mast cells, langerhans and dentritic cells are demonstrated.
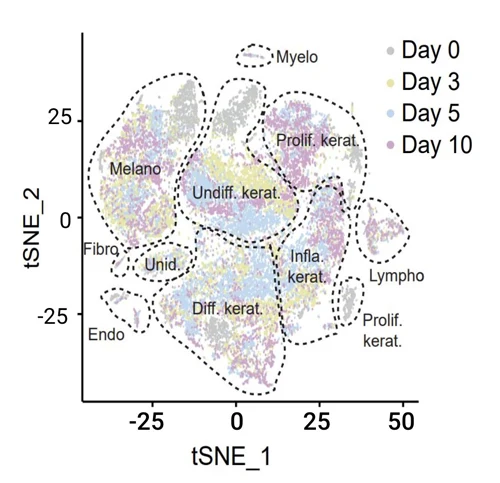
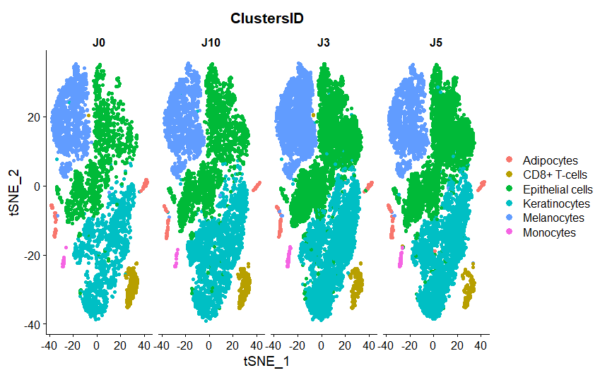
The figure on the right illustrates the presence of the various cell populations present in the skin over 10 days of culture.
Functional and activatable skin-resident human immune cells
HypoSkin® remains fully living and immunocompetent throughout a 7-day experimental period. This enables longitudinal secretome analysis for evaluating the toxicity and efficacy of therapeutic and chemical compounds.

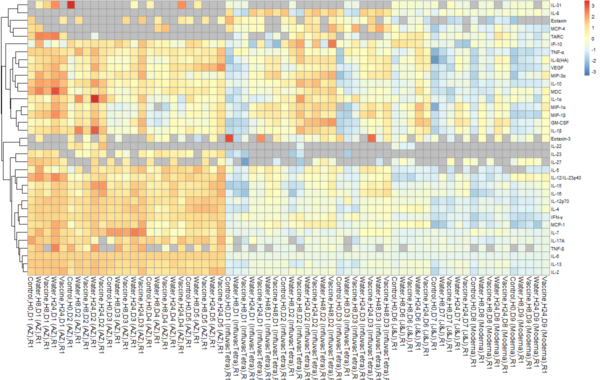
Fold change of cytokines concentrations in clinical-grade water vs vaccine injected HypoSkin® skin model after 8h and 24h in 6 different donors (D=donor).
Injectable human skin model
HypoSkin® is a human skin model that provides the closest substitute for in vivo injection of compounds. Whether your compound is meant to be subcutaneously or intradermally injected, you can use HypoSkin® model to assess it’s efficacy or toxicity.
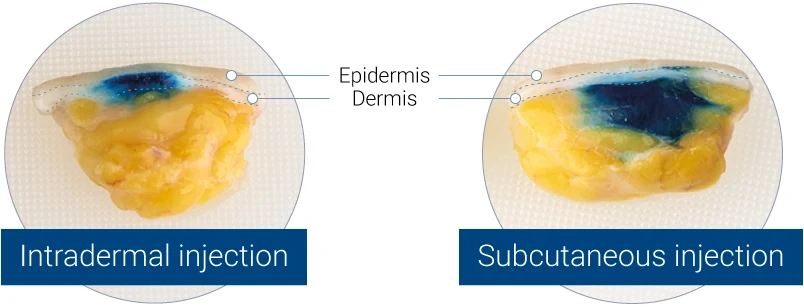
Intradermal (left panel) or subcutaneous (right panel) injection of blue dye into HypoSkin® human skin models. Split views of the model are presented.
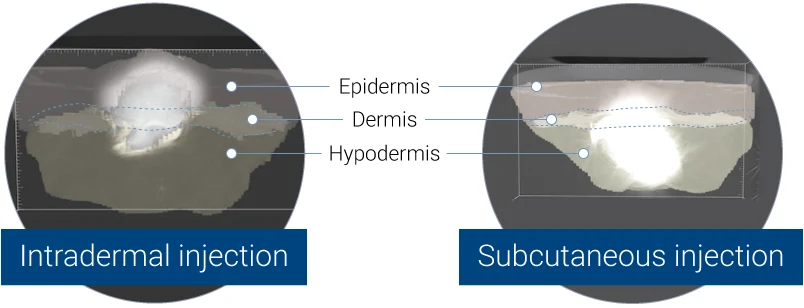
3D representation acquired by X-ray tomography, showing the bolus in the dermis (left panel) or in the hypodermis (right panel).
Cutting-edge data analysis for relevant results
Get the whole picture before you move forward

To help you obtain the full picture of how skin and immune cells respond to your compound and formulation, our expertise includes innovative data generation strategies integrated with unbiased data analysis thanks to proprietary bioinformatic tools.
Scientific publications using HypoSkin®
See how others use the HypoSkin® model to generate results

June 2024 - Investigating Snake-Venom-Induced Dermonecrosis and Inflammation Using an Ex Vivo Human Skin Model.
Published by Toxins – June 17, 2024. Toxins 2024, 16(6), 276; https://doi.org/10.3390/toxins16060276.
Jaffer Alsolaiss, Gail Leeming, Rachael Da Silva, Nessrin Alomran, Nicholas R. Casewell, Abdulrazaq G. Habib, Robert A. Harrison and Cassandra M. Modahl.
August 2022 - Immortalized human myoblast cell lines for the delivery of therapeutic proteins using encapsulated cell technology
Immortalized human myoblast cell lines for the delivery of therapeutic proteins using encapsulated cell technology
Published in Molecular Therapy Methods & Clinical Development – VOLUME 26, P441-458, SEPTEMBER 08, 2022
Aurelien Lathuiliere, Remi Vernet, Emily Charrier, Muriel Urwyler, Olivier Von Rohr, Marie-Claude Belkouch, Valentin Saingier, Thomas Bouvarel, Davy Guillarme, Adrien Engel, Patrick Salmon, Thomas Laumonier, Julien Grogg, Nicolas Mach
November 2021 - A suspended layer additive manufacturing approach to the bioprinting of tri-layered skin equivalents
A suspended layer additive manufacturing approach to the bioprinting of tri-layered skin equivalents
Published in APL Bioengineering – 2021 Nov 30;5(4):046103.
Richard J A Moakes, Jessica J Senior, Thomas E Robinson, Miruna Chipara, Aleksandar Atansov, Amy Naylor, Anthony D Metcalfe, Alan M Smith, Liam M Grover
July 2021 - Poster - Mitigation of Injection Site Reactions after Subcutaneous Administration of Dalcinonacog Alfa (DalcA) in Hemophilia B Using Preclinical Models
Mitigation of Injection Site Reactions after Subcutaneous Administration of Dalcinonacog Alfa (DalcA) in Hemophilia B Using Preclinical Models
Scientific Poster – ISTH 2021 Congress – Abstract PB0453
N. Le Moan, L. Kelly, E. Merle, P. Descargues, N. Gaudenzio, H. Gagnon, A. Chatterji, G.E. Blouse
July 2021 - Poster - Assessing subcutaneous injection site reactions by leveraging immunocompetent human skin and artificial intelligence
Assessing subcutaneous injection site reactions by leveraging immunocompetent human skin and artificial intelligence
Scientific Poster – CRS 2021 Virtual Annual Meeting – July 25 to 29, 2021.
Emeline Pagès, Emilie Braun, Eric Merle, Pascal Descargues, Nicolas Gaudenzio
December 2020 - Standalone or combinatorial phenylbutyrate therapy shows excellent antiviral activity and mimics CREB3 silencing
Standalone or combinatorial phenylbutyrate therapy shows excellent antiviral activity and mimics CREB3 silencing
Published in Science Advances – 2020 Dec 4;6(49):eabd9443
Tejabhiram Yadavalli, Rahul Suryawanshi, Raghuram Koganti, James Hopkins, Joshua Ames, Lulia Koujah, Aqsa Iqbal, Krishnaraju Madavaraju, Alex Agelidis, Deepak Shukla
August 2019 - Near-infrared light activable hydrogels for metformin delivery
Near-infrared light activable hydrogels for metformin delivery
Published in Nanoscale – 2019 Aug 29;11(34):15810-15820
Li Chengnan, Quentin Pagneux, Anna Voronova, Alexandre Barras, Amar Abderrahmani, Valérie Plaisance, Valerie Pawlowski, Nathalie Hennuyer, Bart Staels, Lea Rosselle, Nadia Skandrani, Musen Li, Rabah Boukherroub, Sabine Szunerits
December 2018 - Poster - A fully functional ex vivo human skin model to study human skin microbiome
A fully functional ex vivo human skin model to study human skin microbiome
Scientific Poster presented at the BacTouBac meeting of the Paul Sabatier University in Toulouse – December 11 & 12, 2018
B. Coupe (Vaiomer), M. Pastore (Genoskin), E. Pagès (Genoskin), E. Braun (Genoskin), A. Broha (Vaiomer), P. Descargues (Genoskin)
January 2018 - Poster - Evaluation of local inflammatory reactions following subcutaneous injection of a pro-inflammatory cocktail in a fully human ex vivo skin model
Evaluation of local inflammatory reactions following subcutaneous injection of a pro-inflammatory cocktail in a fully human ex vivo skin model
Scientific Poster presented at DECHEMA Advances in Medical Biology Seminar – January 30 to 31, 2018
C. Jardet (Genoskin), E. Pagès (Genoskin), E. Raude (Genoskin), F. Seeliger (AstraZeneca), L. Brandén (AstraZeneca), E. Braun (Genoskin), M. Ingelsten (LAAS-CNRS), P. Descargues (Genoskin)