Local tolerance studies
Assess local human skin toxicity ex vivo
Local tolerance studies
Assess local human skin toxicity ex vivo
Unlock your local tolerance studies with non-clinical human-centric platforms

Non-clinical local tolerance testing is a fundamental component in the evaluation of human therapeutics, ensuring the safety of both active substances and excipients at contact sites within the body following clinical use. Regulatory agencies, like the European Medicines Agency (EMEA)1 or the Food and Drug Administration (FDA)2, provide essential guidance to underscore the critical role these tests play in pharmaceutical and biotech industries.
Uncover the latest advancements in local tolerance testing services.
Specialized for evaluation of cytotoxicity and local tolerance, our innovative HypoSkin® ex vivo platform ensures comprehensive evaluation of therapeutic candidates, unveiling critical insights without compromising human or animal welfare.
Cytotoxicity Assessment Assays

Explore the tissue responses with our customizable testing services. We employ a variety of robust assays such as:
HEMATOXYLIN AND EOSIN (H&E) STAINING
Uncover detailed histological insights through evaluation of cellular and tissue architecture, and identification of necrotic or inflamed tissue.
TUNEL ASSAYS
Identify apoptotic cells through the detection of DNA fragmentation with TUNEL assays, to evaluate the cellular health.
INFLAMMATION
Assess the impact of treatment on local inflammation using multiplex cytokine analysis & a bioinformatics-based analytical approach.
High-resolution macroscopic imaging
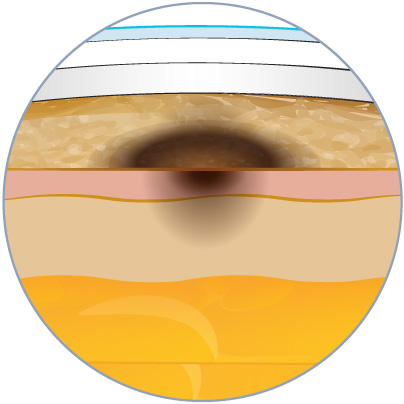
Macroscopic visualization of tissue integrity is crucial in evaluating early toxicity-related reactions at the injection site. This visual insight (skin darkening or coloring) is essential for identifying potential adverse effects, e.g. necrosis, and guiding subsequent analyses to elucidate the underlying mechanisms. Understanding the early signs of toxicity allows for timely intervention and the development of strategies to mitigate potential risks, ensuring the safety and efficacy of the injected substance or treatment.
Sequential macroscopic images at D0, D1, and D3 post-injection. Noticeable skin darkening in HypoSkin models begins at D1, becoming more extensive by D3.
Tissue structure
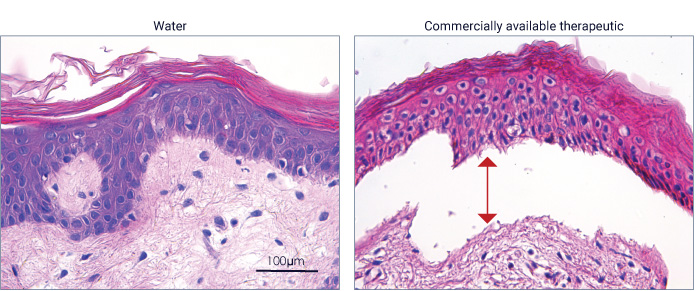
Histological assessment and evaluation of cellular and tissue architecture with hematoxylin and eosin (H&E) staining to identify areas of necrosis or inflammation.
Subcutaneous injection of water and commercially available compound at D0. Collection of skin samples at D5. Histological assessment after H&E staining of samples shows separation of the epidermis at the epidermal-dermal junction.
Cell death
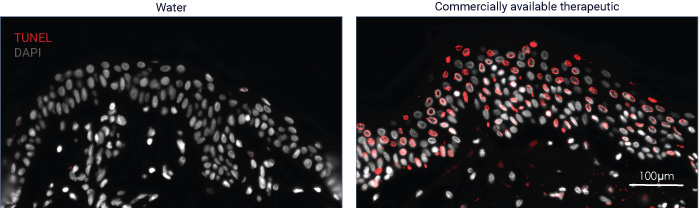
Utilizing TUNEL assays, DNA fragmentation is detected to identify cells death, offering a valuable method for the precise identification of this crucial cellular process.
Understanding a drug’s influence on cell death is crucial for evaluating its cellular impact, including mechanisms of action and potential off-target effects. This understanding not only aids in assessing drug safety profiles but also in fine-tuning therapeutic efficacy. By analyzing cell death pathways, we can discern critical insights into drug-induced cytotoxicity and immunogenic cell death, facilitating the optimization of dosage and administration strategies while minimizing undesirable outcomes.
Subcutaneous injection of water and commercially available compound at D0. Collection of skin samples at D5. TUNEL assay shows DNA breaks in the epidermis. Cells undergoing DNA fragmentation are shown in red.
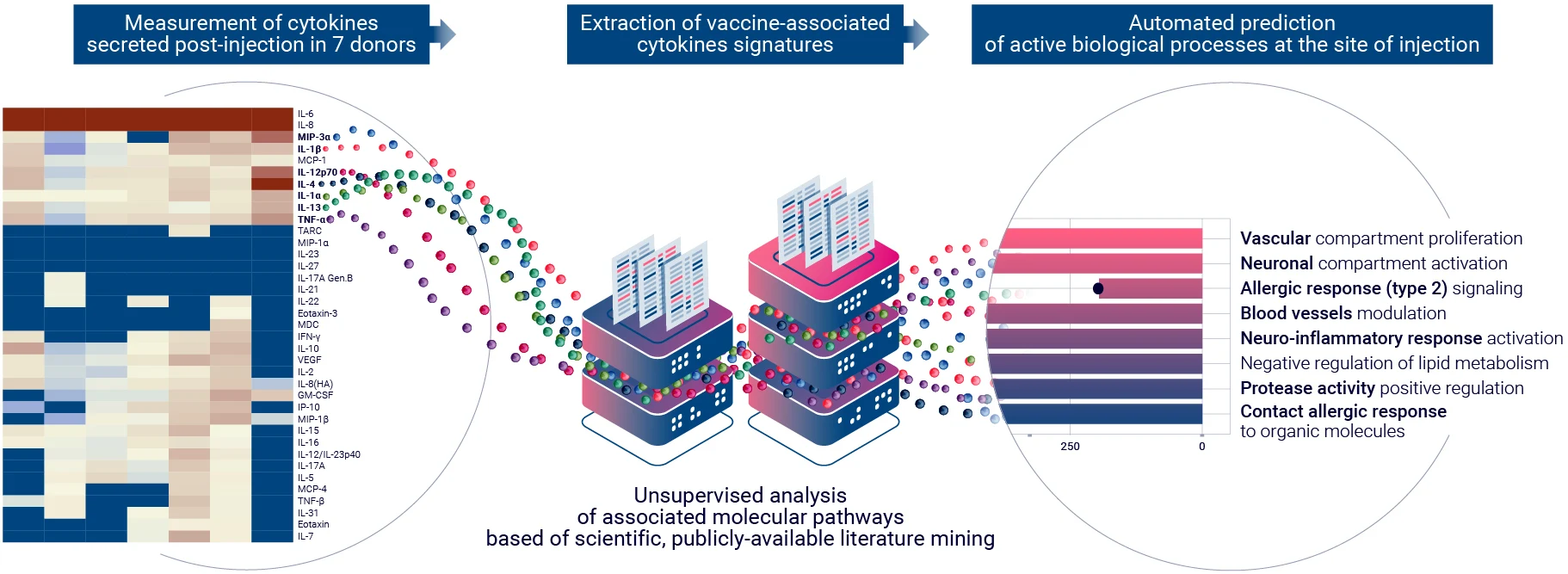
Inflammation
Assess the impact of treatment on local inflammation using multiplex cytokine analysis and a bioinformatics-based approach through the proprietary AUDACY application.
Unlock biological pathway insights when selecting seven different donors, diving deeper into mechanism of action of your therapeutic candidate. Acquire relevant human data before filing an IND.
Why Choose Our Local Tolerance Testing Services?

PRECISION
Leverage the precision of HypoSkin®, which embodies the complex biological diversity and reactivity inherent in human skin. Rather than merely replicating, it preserves the authentic characteristics of human skin immune system and metabolism.
COMPREHENSIVE INSIGHTS
Take advantage of a suite of assays that offer a multifaceted view of cytotoxic effects. Our study directors design study experiments according to your specific needs.
ETHICAL INNOVATION
Conduct advanced toxicity testing with peace of mind, prioritizing ethical considerations with human ex vivo data.