HypoSkin®, an ex vivo human skin model to predict toxicity & efficacy of subcutaneous drugs
Recent advancements in subcutaneous injection testing have brought about significant improvements in drug delivery and patient experience. These advancements include the development of innovative injection devices and formulations that enhance the ease and comfort of subcutaneous drug administration. Market trends also indicate a growing demand for subcutaneous injection therapies due to their effectiveness and convenience.
Furthermore, ex vivo models are playing a crucial role in reducing drug development costs and improving predictive accuracy. By simulating human physiological conditions outside the body, ex vivo models allow for more accurate testing of drug formulations and their interactions with biological systems. This reduces the need for costly and time-consuming animal model studies while enhancing the likelihood of success in clinical trials.
HypoSkin® is the first highly predictive and ethical ex vivo human skin model containing normal subcutaneous tissue architecture. This model enables us to accurately evaluate and optimize a new compound or formulation after subcutaneous injection during the early stages of product and drug development.
HypoSkin®: a unique ex vivo human skin model for subcutaneous injection testing
HypoSkin® consists of all three layers of the skin: the epidermis, dermis, and hypodermis, also known as subcutaneous or fat tissue. Building on this foundation, Genoskin has developed a unique, patented biological matrix that allows for maintaining normal cell viability and the 3D structure of the subcutaneous tissue model for seven days. Genoskin’s team of skin experts has demonstrated the structural biostabilization of the model using Hematoxylin & Eosin and immunofluorescence staining as well as biphoton imaging. Results show that all three skin layers maintain their integrity for over seven days. Genoskin also showed through multiplex imaging and single-cell RNA sequencing that the HypoSkin® model sustains the maintenance of all skin-resident immune cells for over a week. Finally, the immunocompetency of the model was highlighted by analyzing HypoSkin®’s secretome following vaccine injection.
Moreover, the model is robust and malleable enough to withstand the injection of a large volume (up to 125 µL) of liquid formulation into the adipose tissue. It is also possible to perform intradermal injections and to test various medical devices such as drug delivery systems.
This patented technology is currently offered as a standardized, ready-to-use testing kit and as a service that can be customized to meet customers’ specific needs.
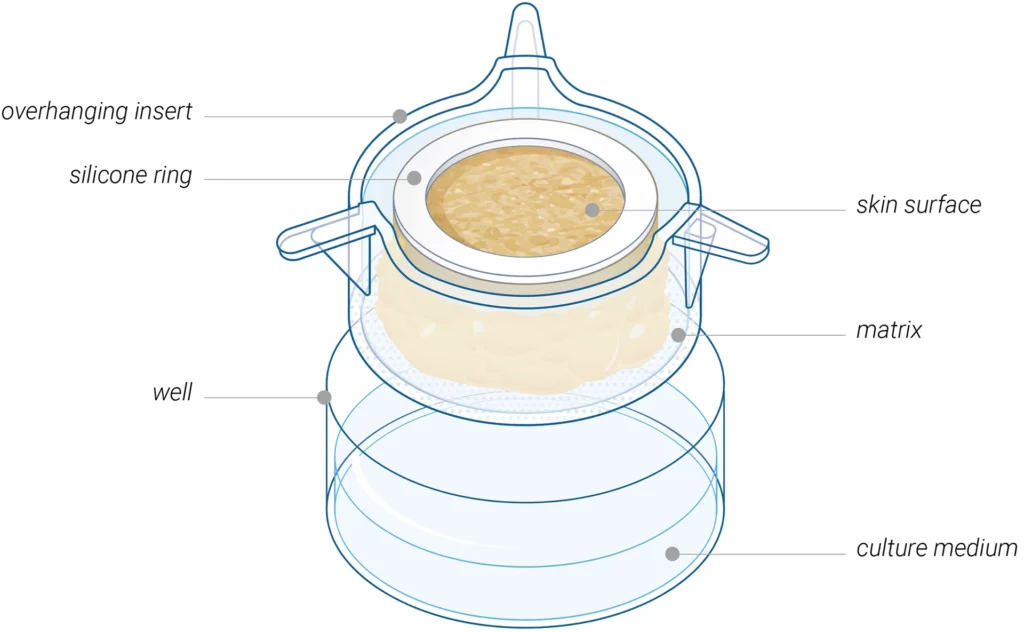
Genoskin’s unique HypoSkin® technology model
A patented technology developed for a wide range of applications
HypoSkin® human skin models currently offer the closest alternative to directly injecting a compound into an actual person’s skin. We designed the model to help drug and vaccine developers accelerate the selection of viable drug candidates. HypoSkin® is also suitable for a wide range of applications including:
- Drug-induced toxicity studies: Assessment of the safety profile of drugs by analyzing their potential to cause adverse reactions.
- Drug metabolism studies: Evaluation of drug processing within human skin, providing essential pharmacokinetic data.
- Biodistribution studies: Tracking the distribution and localization of substances after their administration to better understand therapeutic effects and off-target interactions.
- Vaccine development: Evaluation of the immunogenicity and efficacy of vaccines by simulating their administration and observing immune response in human skin.
- Bolus of injection analysis: Study of the immediate effects of injection volume and formulation on skin physiology and drug delivery efficiency.
- Medical device testing: Evaluation of the compatibility and safety of medical devices that interact with skin, such as patches and injection systems.
- Dermal filler characterization: Analysis of the behavior and impact of dermal fillers within human skin to assess performance and potential side effects.
- Testing of therapeutics against infectious diseases: Investigate how pathogens affect human skin by mimicking natural infection processes, and evaluate the efficacy of therapeutics against pathogens.
- Radiochemical studies: Examine the skin’s response to radiochemical exposure, crucial for understanding absorption, metabolism, and biological effects to provide mechanistic insights into the interactions between radiochemicals and skin components.
Validated support to guide you through your HypoSkin® experiments
Genoskin offers comprehensive tools to assist customers in initiating their own HypoSkin® studies. The User Manual includes detailed descriptions of the HypoSkin® model, available formats for various administrations, and a step-by-step guide for kit setup, compound administration, culturing, and biopsy collection. For additional support, Genoskin provides protocols on subcutaneous and intradermal injections, along with downloadable analysis protocols.
Customers can also access expert consultations for personalized assistance and in-person training sessions. Additionally, Genoskin shares a list of peer-reviewed publications from early adopters using HypoSkin® models.
Useful links
- Download HypoSkin® User Manual
- Download Subcutaneous Injection Protocol
- Download Intradermal Injection Protocol
- Request a meeting with our expert team
Comments are closed.





