Human-based pilot study for early local toxicity assessment
De-risk your injectable therapeutics with a New Approach Methodology

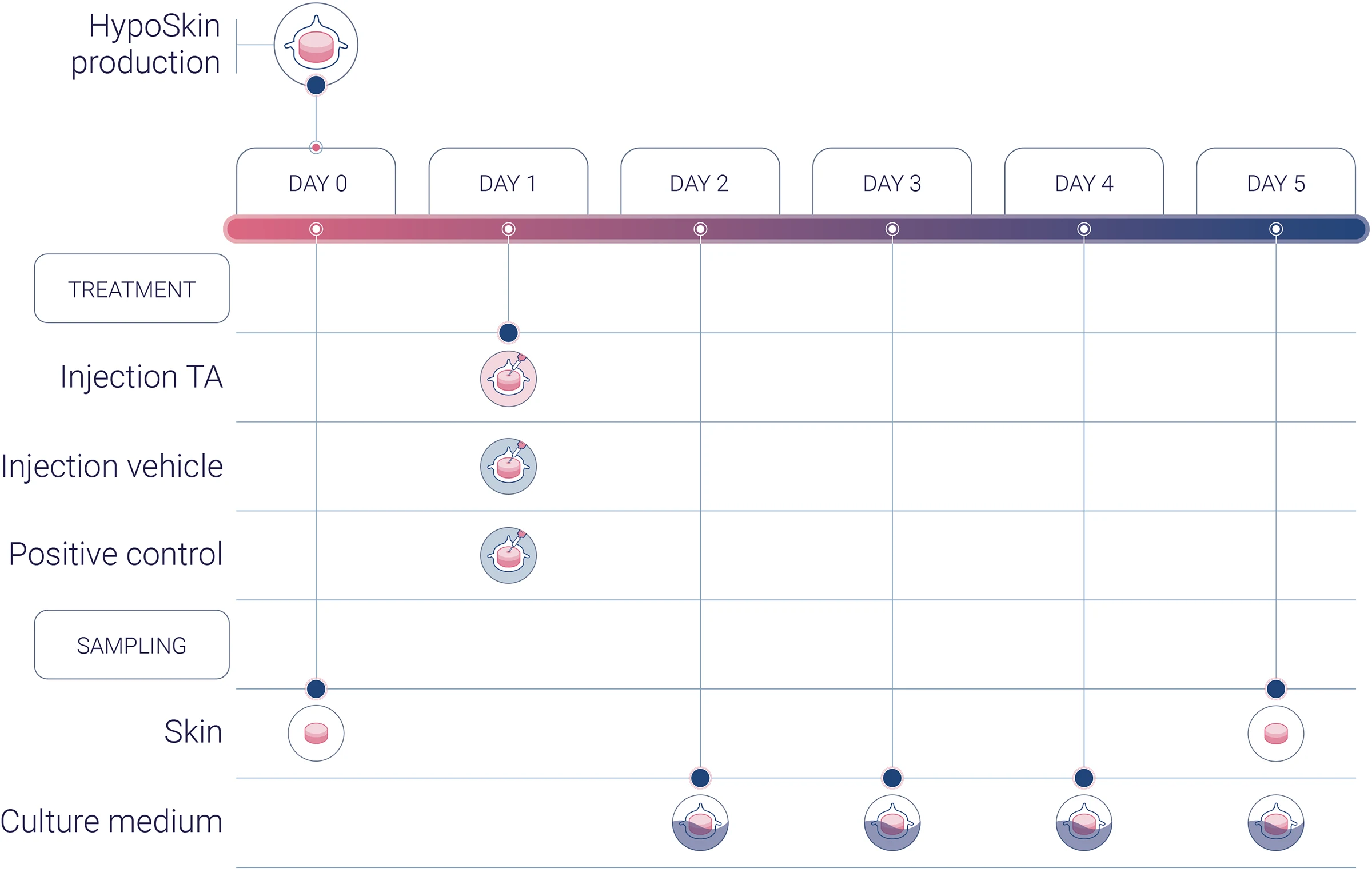
Experimental conditions
1 donor (n=3)
1 vehicle (negative control)
1 TA
1 positive control
Analysis
H&E staining
Multiplex cytokine analysis
Evaluate local toxicity using biostabilized human skin
Study overview

Objective of the study
Evaluating local toxicity and injection site reaction in human skin is essential to de-risk injectable therapeutics before moving to clinical stages. Genoskin’s HypoSkin® platform provides an ex vivo, immunocompetent human skin model that preserves native structure, innate immune components, and subcutaneous architecture – delivering human-relevant insights that align with current NAM-focused regulatory expectations.
This toxicity pilot study offers an entry point to assess whether your therapeutic compound elicits morphological damage or initiates early inflammatory responses when injected into human subcutaneous tissue. Based on ex vivo biostabilized human skin, this New Approach Methodology provides decision-enabling data that support early safety assessment and guide downstream development.

This feasibility study is recommended when:
You are new to Genoskin’s technology and want to validate its relevance to your program.
You need early human-based data to support internal decision-making.
You require preliminary evidence to justify a larger, multi-donor investigation.
You are looking for preliminary data before setting-up a larger program to de-risk several therapeutic candidates
Analysis & readouts
Tissue viability: Hematoxylin & Eosin (H&E) staining.
Immune response assessment: Multiplex cytokine analysis
Specific questions addressed in the pilot study
Does the compound alter epidermal or dermal morphology following injection?
Does the compound trigger cytokine release relative to vehicle control?
Are early immune signals detectable between days 2–5 post-injection?
Are culture media cytokine trends supportive of further investigation or a change in readouts?
Experimental workflow

Deliverables & important information

Deliverables included in the pilot study
- Immunohistochemistry (H&E) image files
- Cytokine analysis datasets
- Presentation-ready results
- Raw data available on demand
Final report:
- Full data interpretation
- Delivered as a slide deck
- Alternate formats available upon request prior to study approval
Biological variability
A pilot study is designed as an initial feasibility test to observe how your molecule behaves in humannskin and to help identify the most relevant readouts for assessing local toxicity. For statistically robust and reliable conclusions, a follow-up study should be planned with multiple donors and an appropriate number of models per endpoint.
Scope & limitations of use
Use of the materials excludes:
- Any clinical and/or medical applications
- Attempts to discover composition, manufacturing processes, or trade secrets behind the materials
Ethical sourcing & compliance
Full terms and conditions can be accessed here: Terms & conditions.
Request your quote
Comments are closed.