Brand Identity
What I Learned In A French Lab That Led To My Role As A CEO
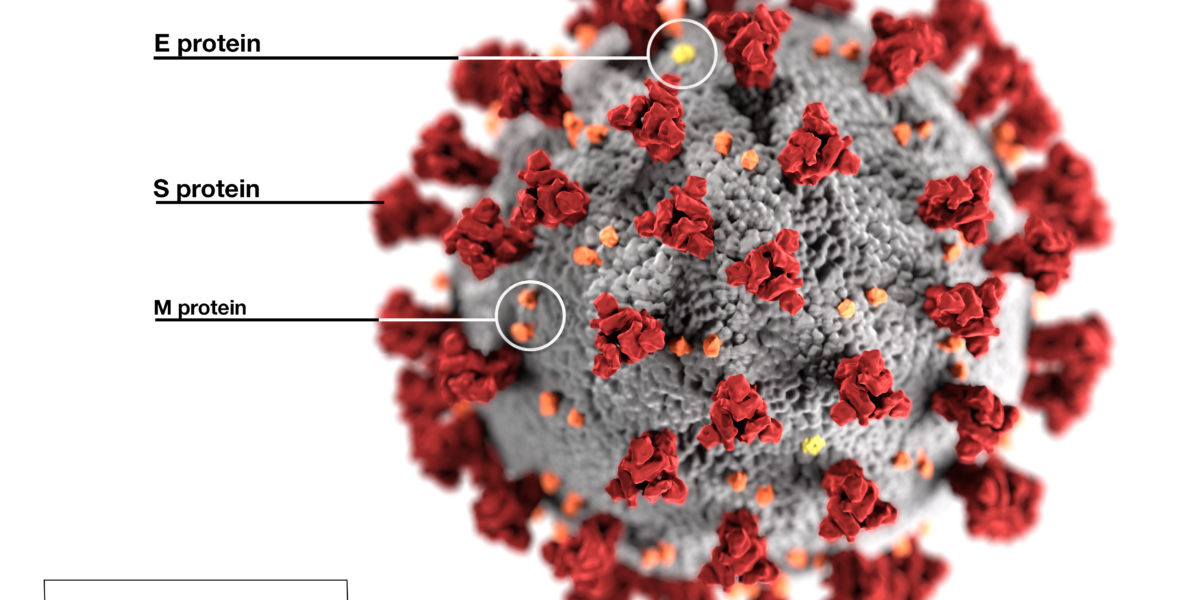
Genoskin supports CoViD19 research!
Genoskin COVID-19 research preferred access program On Friday, March 13th 2020, Genoskin launched a preferred access program for all research teams actively developing therapeutic and vaccine technologies for the COVID-19 pandemic. The World Health Organization (WHO) officially declared on March 11th, the outbreak of COVID-19 a pandemic. The disease caused by SARS-CoV-2 has spread globally. Over 130,000 individuals are affected so
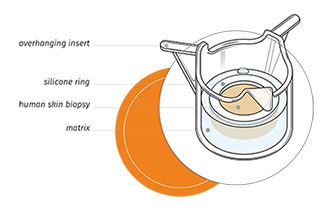
US Patent for human skin models
Genoskin obtains US patent for skin models Genoskin is pleased to announce the approval of a US patent for our “system for the maintenance and survival of skin biopsies in transport and its applications”. The patent describes our method to transform human skin biopsies in ready-to-use and functional ex vivo skin models. The human skin biopsies that are used in our models