Genoskin launches new skin offering, NativeSkin® access, that increases accessibility to animal testing alternatives
NativeSkin, the only ex vivo human skin model sold online, will be available to purchase daily at a key price point, making it easier to order even for larger volume sales
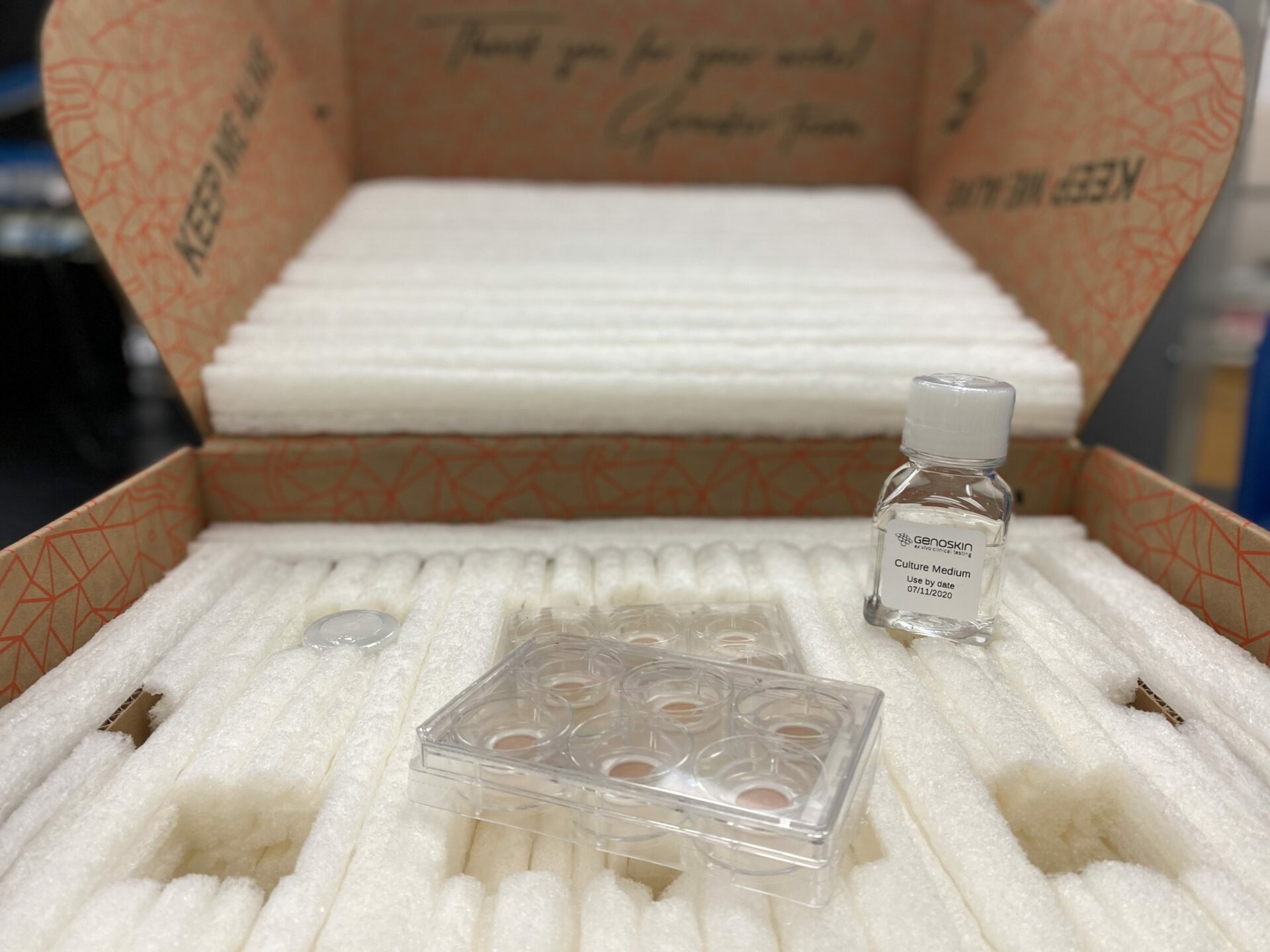
Toulouse, France & Salem, MA, USA, September 1, 2020 – Genoskin, a biotechnology company developing ready-to-use human skin biopsies for ex vivo clinical testing, announces today the launch of its newest offering, NativeSkin® access, a ready-to-use standardized skin model.
This skin biopsy will be embedded in Genoskin’s proprietary gel-like matrix, where it can be maintained alive and functional for at least seven days. Ready-to-use and standardized models will be available on-demand and sold in kits with a culture medium. Genoskin’s production units are located in Europe and in the US; the product will reach customers worldwide within a few days.
“Our amazing teams of skin experts responded to researchers’ calls for an easily accessible, less expensive ex vivo human skin model to be conveniently sold online. We are excited to offer NativeSkin access models that are fully functional, immunocompetent and better performing than competing products,” said Pascal Descargues, Ph.D, CEO of Genoskin.
NativeSkin access will be sold globally in two sizes. Both 8-millimeter and 11-millimeter diameter models will be sold online for $99 in US markets and €99 in European, Asian, ROW markets, with no minimum order. This product is an accurate, safe, sustainable and accessible alternative to animal testing, a clinical method now banned in many countries, such as within the EU and in India.
NativeSkin access allows researchers to test efficacy, absorption and toxicity of topical compounds. They can also study the effects of UV and pollution exposure, immune response, metabolism, melanogenesis, barrier function and regeneration.
“Since 2011, Genoskin has led the field in offering the only live ex vivo human skin platform on the market. We are advancing our technology and models with the launch of NativeSkin access, the only human skin model to be sold online. We believe this great step forward will revolutionize clinical testing. It will enable companies to test compounds on living, ready-to-use skin assays at a faster rate and at a price aligned with that of reconstructed and bio-printed tissue,” added Dr. Descargues.
Genoskin’s NativeSkin models are covered by US patent US9585381B2. To date, there are no equivalent skin models on the market.
We are happy to also speak with you and give you more information on our activities. To keep up-to-date with Genoskin’s latest news, follow us on Twitter and LinkedIn.
Comments are closed.