A translational nonclinical service reproducing IL-4/IL-13–driven barrier impairment to support early decision-making in topical atopic dermatitis development.

This service uses native human skin exposed to type-2 cytokines (IL-4 / IL-13) to reproduce key features of atopic dermatitis–associated barrier dysfunction. It enables the evaluation of topical therapeutic candidates based on their ability to:
- Normalize disease-relevant molecular pathways
- Modulate AD-associated markers in a preserved human tissue context
The service is designed to support lead selection, compound ranking, and formulation decisions in topical atopic dermatitis programs.
Atopic dermatitis: a type-2–driven inflammatory skin disease with barrier dysfunction
Atopic dermatitis (AD) is a common chronic inflammatory skin disease, affecting a large proportion of children and adults worldwide. Its pathogenesis involves the interaction of immune dysregulation, neuroinflammation, and epidermal barrier dysfunction.
Immunologically, AD is predominantly driven by type-2 (Th2) and Th22 inflammation, with contributions from other immune pathways depending on disease stage and patient subtype. The central role of type-2 inflammation is highlighted by the clinical efficacy of therapies targeting this axis, such as IL-4/IL-13 pathway inhibition(1).
A defining feature of AD is skin barrier dysfunction, resulting from complex interactions between keratinocytes and tissue-resident immune cells, including T helper cells, innate lymphoid cells, mast cells, and basophils. These cells produce type-2 cytokines (notably IL-4 and IL-13), which profoundly alter epidermal biology.
Type-2 cytokine signaling disrupts the expression of genes involved in epidermal differentiation, lipid metabolism, and cell–cell junctions, weakening the barrier and increasing susceptibility to microbial colonization. Together, these processes drive the structural and functional abnormalities characteristic of atopic dermatitis(2).
(1)P. D’Avino et al. Distinct Roles of IL-4, IL-13, and IL-22 in Human Skin Barrier Dysfunction and Atopic Dermatitis. Allergy Volume 81, issue 2, 02.2026. https://doi.org/10.1111/all.70060
(2)Lisa A. et al. Type 2 Inflammation Contributes to Skin Barrier Dysfunction in Atopic Dermatitis, JID Innovations, Volume 2, Issue 5, 2022, 100131, ISSN 2667-0267, https://doi.org/10.1016/j.xjidi.2022.100131.
A cytokine-driven human skin model reproducing atopic dermatitis-like alterations
The model enables quantitative assessment of therapeutic impact across three complementary dimensions.

By P. D’Avino et al. Distinct Roles of IL-4, IL-13, and IL-22 in Human Skin Barrier Dysfunction and Atopic Dermatitis. Allergy Volume 81, issue 2, 02.2026.
Type-2 driven inflammation protein markers in ex vivo human skin
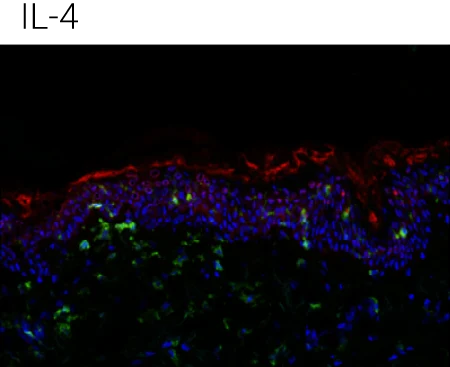
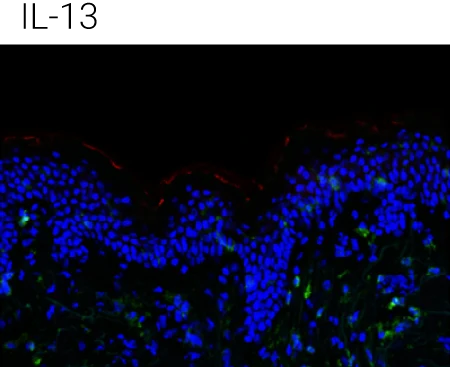
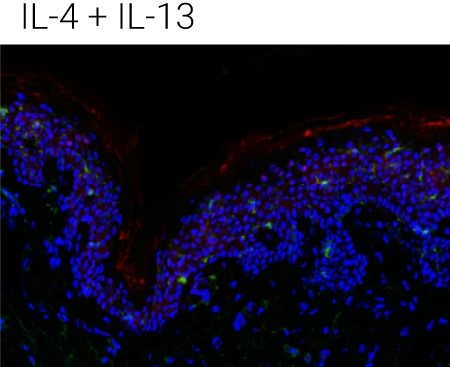
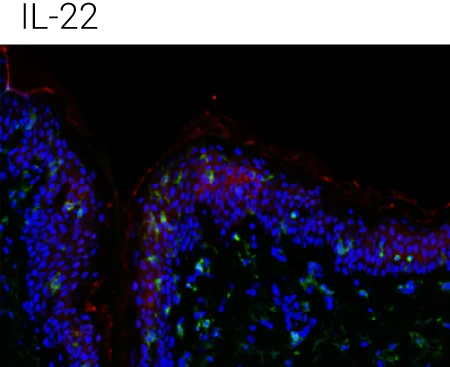
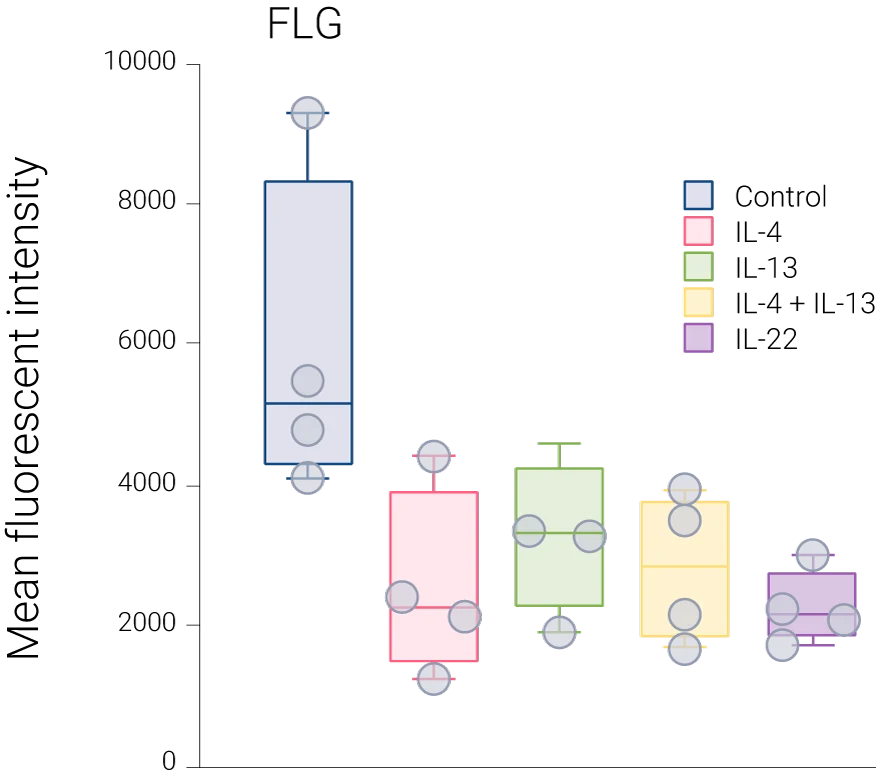
Reduced barrier protein expression: FLG protein levels are significantly decreased following IL-4, IL-13, and IL-4+IL-13 stimulation compared to control, confirming barrier impairment at the protein level.
IL-22 shows a distinct profile: IL-22 also reduces barrier integrity but is particularly associated with epidermal stress and antimicrobial peptide programs, supporting its complementary (non-redundant) role in AD pathology.
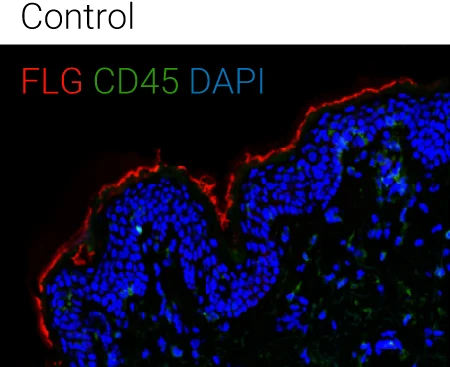
Immune compartment preserved: CD45⁺ cells remain detectable in the dermis, indicating that cytokine stimulation alters barrier biology without destroying tissue viability or immune readouts.
Representative immunohistochemistry images of FLG (red), CD45 (green), and DAPI (blue). FLG expression was evaluated with CD45+ cells in the ex vivo skin upon the different stimulations (control,IL-4 + IL-13, n = 4 biological donors). Box plots comparing the mean fluorescent intensity of FLG expression. (n = 4), ONE way ANOVA, *p < 0.05.
Type-2 cytokine–driven transcriptomic remodeling
Bulk RNA-seq identifies differentially expressed genes and pathways associated with:
- Epidermal differentiation
- Barrier function
- Type-2 inflammatory signaling
Here, to analyze the effects of the combination of IL-4 and IL-13 on the human skin transcriptome, RNA was isolated after 24 h of stimulation and in order to perform bulk RNA sequencing analysis.
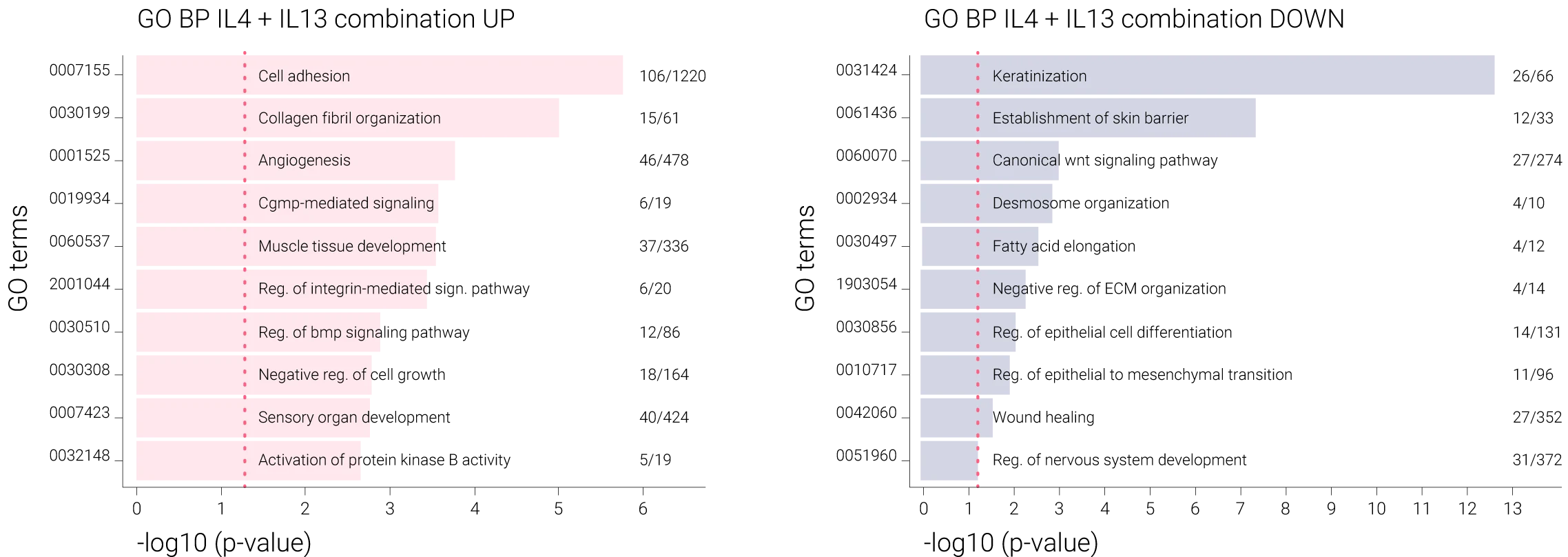
746 upregulated and 798 downregulated genes were significantly modulated by the combination of IL-4 and IL-13. Through this transcriptomic analysis, specific genes were identified to be stimulated or suppressed by the combination of IL-4 and IL-13. In addition, the differentially expressed genes (DEGs) from each sample were subjected to GO term enrichment analysis to identify the biological processes altered by these cytokines.
Samples stimulated with a combination of IL-4 and IL-13 exhibited a strongly enriched gene expression of pathways involved in AD pathogenesis. In contrast, there was a suppression of genes belonging to barrier-related pathways such as keratinization, establishment of the skin barrier, desmosome organization, and regulation of epithelial cell differentiation, including CLDN1, CLDN4, KRT1, and CDSN.
Comparison of DEG of IL-4 + IL-13 induced models vs controls (no induction). Numbers = numbers of overlapping significantly DEG/# of genes involved in process.
Functional assessment of epidermal barrier integrity
- Electrical impedance spectroscopy (EIS) quantifies barrier disruption induced by IL-4 / IL-13
- Time-resolved measurements capture kinetics of barrier impairment and recovery
- Treatment effects translate into a functional readout, directly relevant to topical development
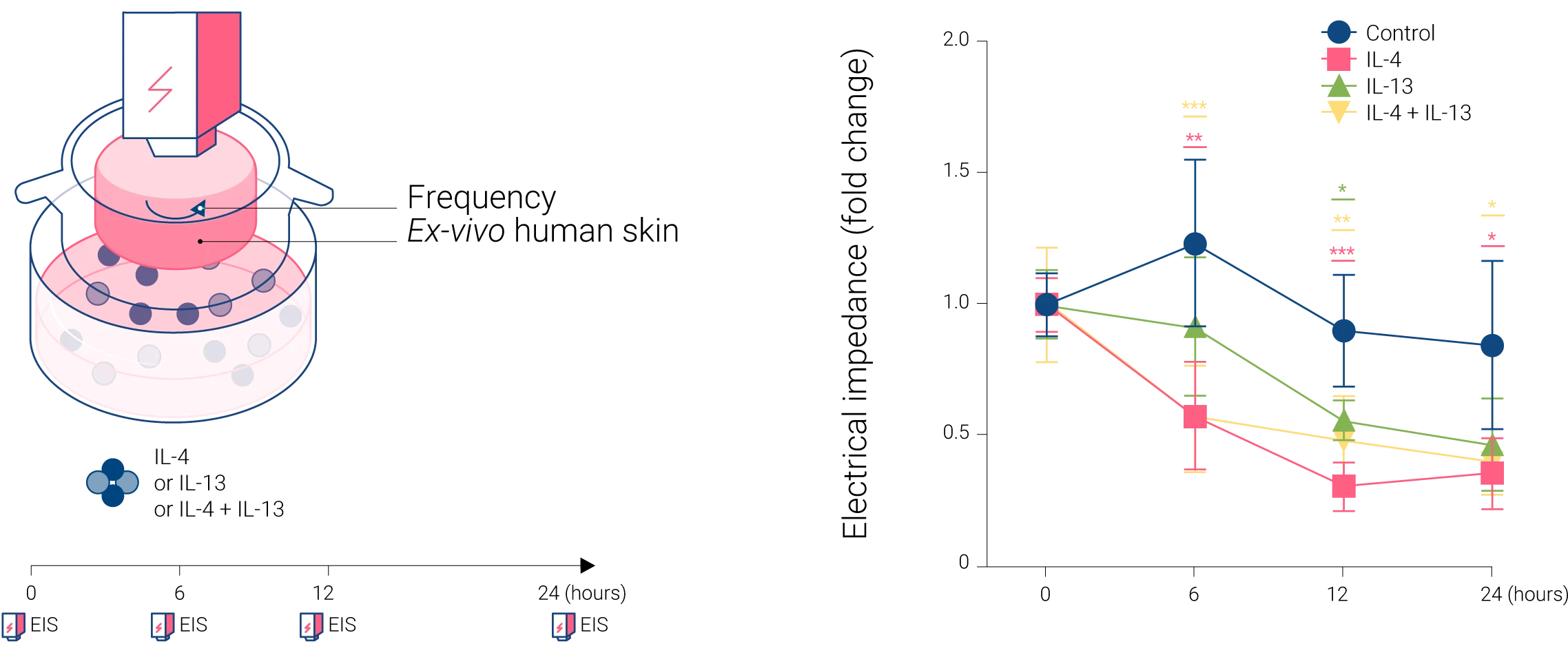
Type-2 cytokine exposure decreases electrical impedance and downregulates the expression of skin barrier-related genes in ex vivo human skin.
Ex vivo models were treated with IL-4, IL-13, and their combinations. EIS measurements were recorded before treatment and at various time points (Figure A). As shown in Figure B, after 6 h, IL-4 and the combination of IL-4 and IL-13 significantly reduced the electrical impedance, and IL-13 alone reached similar values after 12 h. These findings demonstrate the damage induced by IL-4 and IL-13 on the epithelial barrier integrity in ex vivo human skin.
Electrical impedance measurements were performed on 5 donors before and after the cytokine stimulations at indicated time points (0, 6, 12, 24 h). The data were normalized with the values at 0 h in each sample. A two-way ANOVA test was used to compare differences between each stimulation and control at each time point. Data are shown as mean ± SEM. The star colors indicate the statistical significance corresponding to the colors of the stimulations shown in the legend. *: p < 0.05, **: p < 0.01, ***: p < 0.001.
Frequently asked questions
Is this for topicals only?
Do you provide raw data?
Is this meant to replace in vivo?
What questions does this service answer?
- Does my topical candidate restore epidermal barrier function under type-2 inflammatory conditions?
- Does it normalize AD-relevant molecular pathways altered by IL-4 / IL-13 exposure?
- Does it modulate key epidermal and disease-associated markers in native human skin?
Together, these readouts provide human-relevant, decision-ready evidence to support compound ranking, differentiation, and go/no-go decisions.