Research Highlight
Explore our latest publication in Allergy: the story behind the idea and key findings.
The landscape of vaccine research is undergoing an important transformation, thanks to the breakthroughs in mRNA vaccine technology.
This innovation has catalyzed the development of vaccines that are not only highly effective but also rapidly deployable at a large scale. With the increasing number of research institutions, biotechnology, and pharmaceutical companies working on new mRNA vaccine formulations and innovative delivery methods through the skin, the demand for advanced, scalable platforms to comprehensively assess vaccine immunogenicity and safety ahead of clinical trials is substantial.
Using human skin as a window into human immunology
The human immune system is a complex network of specialized cells that are spread throughout the body and evolve over a person’s lifetime, shaped by various environmental triggers. This evolution helps to form personalized immunological memory, equipping each person with tailored defense mechanisms against pathogens they may encounter. The skin, one of our largest organs, is home to a diverse army of immune cells like dendritic cells, macrophages, Langerhans cells, mast cells, and various T cell subsets. These cells work in concert with the skin’s structural components—such as keratinocytes, connective tissue, and blood vessels—to create an intricate and well-organized biological ecosystem. This makes the skin an ideal candidate for investigating human immune responses in a controlled, yet naturally complex environment outside of the body.
HypoSkin®: a unique technology to study vaccine response
While modern lab techniques have made impressive progress in simulating certain features of human skin, they fall short of fully replicating the native organ and the genetic variety seen in the human population. Tools like organ-on-a-chip, artificial skin models, and bioprinting can emulate important cellular behaviors and drug permeability. However, they do not precisely replicate the complex three-dimensional structure, the network of cellular interactions, or the nuanced functioning of the human immune system. Fortunately, the availability of excess human skin from surgeries has given researchers a valuable resource—cultured skin biopsies—to delve deeper into how our bodies react in response to vaccines.
Leveraging HypoSkin®, a unique technology enabling the maintenance of ex vivo human skin in a living, functional, and immunocompetent state for more than seven days, our dedicated team of scientists has been working tirelessly to push the boundaries of vaccine evaluation. Today, we are proud to share our findings with you. Let’s dive deeper into the publication.
About the Research
With this publication, we introduce VaxSkin®, a flexible solution designed to comprehensively monitor the response of the human skin’s ecosystem to vaccines over time. Based on the HypoSkin® technology, VaxSkin® allows a complete analysis of the response to vaccines at both organ and single-cell levels.
To validate the VaxSkin® platform, we subcutaneously injected the commercially available mRNA-1273 COVID-19 vaccine in HypoSkin® and characterized the precise sequential molecular events triggered upon detection of the exogenous substance.
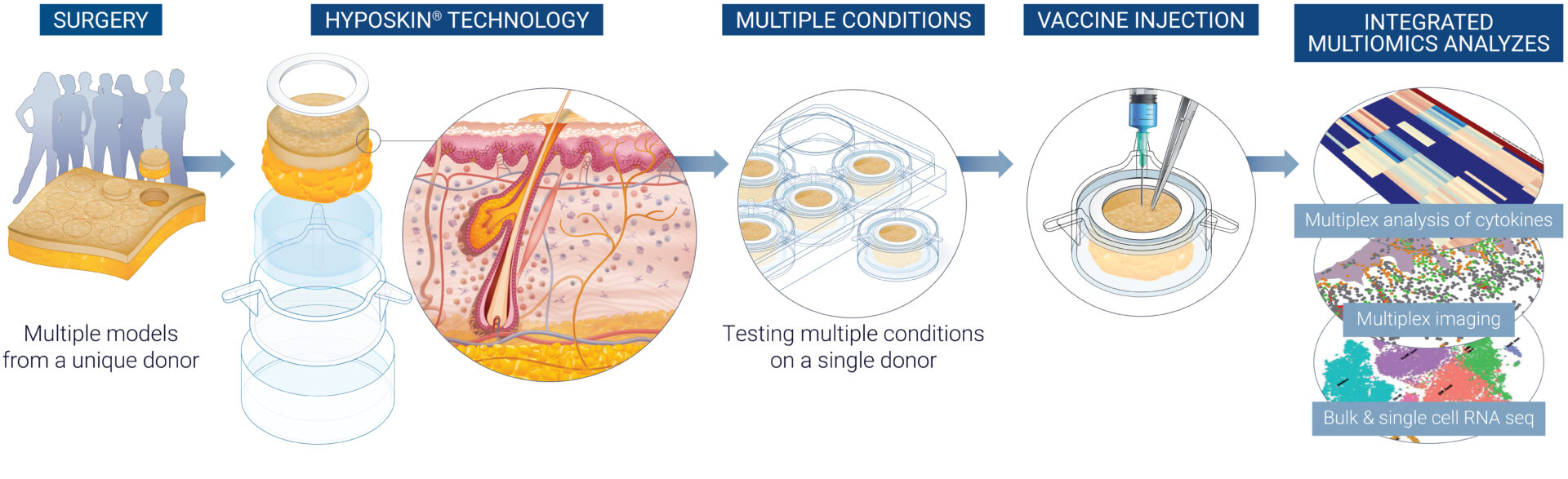
- Quantitative and qualitative validation of reactogenicity in response to mRNA vaccination: Our research, using secretomics, proteomics, and bulk RNA sequencing integrated with a tailored bioinformatic pipeline, revealed a global modulation of the human skin ecosystem following vaccine injection. The research offers valuable insights into how natural human skin adapts and responds, shedding light on the precise sequence of events—from recognizing and responding to a foreign agent to adjusting skin homeostasis, activating stress responses, and initiating immune reactions.
- Single-cell profiling of skin-resident immune cells: Using single-cell RNA sequencing and our proprietary multiplex spatial imaging platform, MANTIS®, we were able to track the sequence of transcriptomic changes involved in effective vaccination, from pathogen sensing to antigen presentation and mRNA transcription. We also showed the relocation of antigen-presenting cells to the dermal area.
Combining these advanced analytical techniques with our proprietary bioinformatic analysis pipeline and our proven HypoSkin® technology, Genoskin offers a comprehensive and flexible solution to evaluate drug immunogenicity and to enable the selection of the most promising vaccine candidate. The research conducted in this publication also demonstrates the adaptability of the platform in assessing the various routes of administration as the subcutaneous and intradermal routes were explored while injecting Moderna’s mRNA-1273 COVID-19 vaccine.
Key Takeways
Our Chief Scientific Officer, Dr. Nicolas Gaudenzio explains how VaxSkin® provides transformational data to help advance vaccine development:
“In humans, vaccine immunogenicity is usually assessed by measuring the detection of antigen-specific antibodies or T cell responses weeks after the injection of the vaccine. However, evaluating the initial stages of the immune response, which include the uptake of vaccines by immune cells and a profound modification of tissue physiological processes, remains challenging in clinical settings, as it would require biopsy procedures at the vaccination site. VaxSkin® enables, for the first time, to evaluate the tropism of a vaccine and how it directly impacts the activation status and migratory potential of human immune cells at the single-cell level in their untouched environment. Moreover, based on secretomic and transcriptomic data of the entire skin tissue, we can also assess whether a vaccine is at risk to generate molecules involved in edema formation and transmission of pain sensation, two classical symptoms associated with vaccination.”
Three key findings from our research on VaxSkin®
- The HypoSkin® model remains bio-stabilized and immunocompetent for more than seven days.
Each HypoSkin® model combines a chemically defined gel matrix with a human skin biopsy and a topical silicone ring. This setup allows for various delivery routes including subcutaneous and intradermal injections using standard clinical syringes while preserving the integrity of native adipose tissue. HypoSkin® enables the simultaneous execution of multiple parallel experiments using a single donor’s sample and facilitates the extension to cohorts that reflect human biological diversity. We compiled all the data about the model validation in a white paper, download it here. - Upon injection of the mRNA-1273 COVID-19 vaccine, we found that the vaccine consistently targets dendritic cells, macrophages, and mast cells, regardless of the administration route. The VaxSkin® platform enables the evaluation of various vaccine administration methods. During our research, we observed that the vaccine administered intradermally has a lasting impact on both myeloid and lymphoid cells compared to the subcutaneous route, which persisted 8h to 24h after post-administration. These insights demonstrate that the vaccine delivery method significantly influences the local immune cell populations within the skin. This effect extends not only to dermal dendritic cells and macrophages but also to a broader spectrum of myeloid and lymphoid cells.
- Given its direct translational relevance, VaxSkin® provides a multiscale vision of skin vaccination that could pave the way toward the development of new vaccination development strategies. Through the analysis of gene expression levels over time, we have been able to map out the sequence of events that unfold between the exogenous substance and the skin biology. This sequence includes the initial detection and reaction to the foreign agent, the adjustments in skin homeostasis, the triggering of stress responses, and the activation of immune responses. In the field of drug and vaccine discovery and development, the VaxSkin® platform emerges as a valuable tool, offering deep insights into the molecular mechanisms at play, helps in prioritizing the most promising lead candidates, and is crucial for toxicity and safety testing.
Read the Full Study
Our research has been published in Allergy, you can read the full study here: Multi-modal profiling of biostabilized human skin modules reveals a coordinated ecosystem response to injected mRNA-1273 COVID-19 vaccine.
If you want a more visual and condensed version of our research, download our poster: Evaluation of Vaccine Potency with High-Dimensional Immune Profiling Using an Innovative Bio-stabilized Human Skin Model.
Let’s Collaborate
We are open to partnerships and collaborations to take this research to the next level. If you are interested, feel free to reach out to us.
Acknowledgments
We would like to extend our deepest gratitude to the Genoskin and Infinity teams for their invaluable contributions to this research. Congratulations Manon Scholaert, Emeline Pagès, Nicolas Gaudenzio, Mathias Peries, Emilie Braun, Jeremy Martin, Nadine Serhan, Alexia Loste, Audrey Bruner, Lilian Basso, Benoit Chapu, Eric Merle and Pascal Descargues.
Comments are closed.