PRESS RELEASE – Salem, MA, USA – October 3rd, 2023
Genoskin introduces ImmunoSafe: ISR Platform® with AUDACY – A revolutionary leap in non-clinical local toxicity assessment
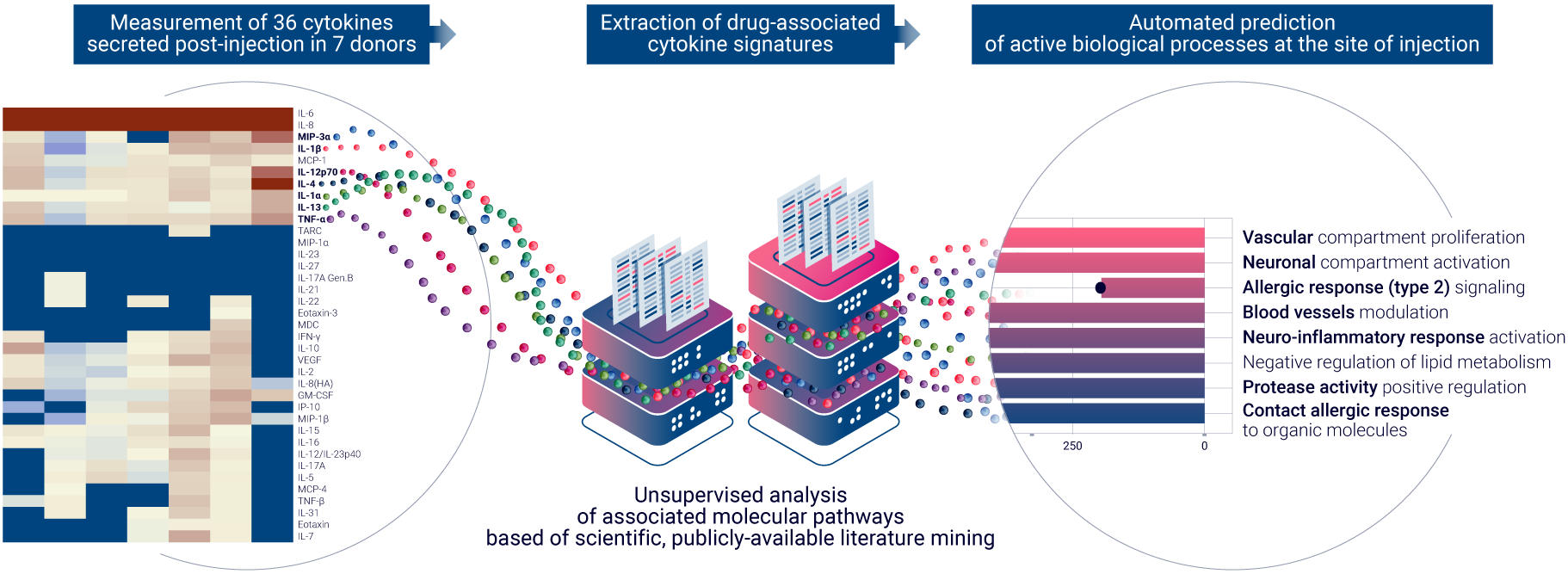
Salem, MA, October 3rd, 2023 – Genoskin, a biotechnology company at the forefront of accelerating drug development, proudly announces the enhancement of its ISR Platform® with the integration of AUDACY, a state-of-the-art bioinformatics-based analytical solution. The new ImmunoSafe: ISR Platform® brings a transformative approach to assessing local toxicity in the realm of non-clinical platforms, specifically focusing on injection site reactions (ISR).
Building on the foundational success of the ISR Platform® launched in 2021, the ImmunoSafe: ISR Platform® now seamlessly combines ex vivo cytokine analysis, cutting-edge computational analysis, and comprehensive literature data mining. This synergy is designed to deduce statistically significant active biological pathways that are activated at the injection site, providing unparalleled insights into the potential unwanted clinical outcomes of therapeutic compounds.
AUDACY, the latest addition to this platform, is engineered to handle vast datasets, ensuring rapid results devoid of human error. By analyzing large datasets, AUDACY identifies proteins that are statistically and differentially expressed, and correlates them with publicly-available literature to infer protein interactions and biological pathway regulation. This unique capability allows Genoskin to offer a more precise evaluation of how therapeutic compounds interact with human immune cells in an ex vivo environment.
Highlighting the significance of this launch, Pascal Descargues, Ph.D., CEO of Genoskin, commented, “The integration of AUDACY into our ImmunoSafe: ISR Platform® services is a testament to our commitment in revolutionizing drug development. By offering a comprehensive solution for local toxicity assessment, we aim to provide our biotech and pharma partners with actionable first-in-human data, ahead of clinical trials, ensuring safer and more effective therapeutic solutions.”
Genoskin recently showcased its innovative approach at Eurotox 23 in September, presenting a poster titled “Automated prediction of drug reactogenicity at the site of injection, by combining natural human skin & computational inference of biological pathway.” This presentation further emphasizes the company’s dedication to advancing the field of non-clinical local toxicity assessment and its potential impact on the future of drug development.
For more information about Genoskin and its pioneering service offering, ImmunoSafe: ISR Platform® with AUDACY, please visit genoskin.com.
About Genoskin
Genoskin provides transformative non-clinical platforms leveraging human skin to test therapeutic and non-therapeutic products. By generating actionable human data, Genoskin provides a reliable alternative to animal experiments, ensuring safer and more effective drug development. The company uses real human tissues, prepared from donated human skin leftovers ethically sourced from plastic surgeries, and innovative technologies to maintain viability, immunocompetency and functionality of the human skin samples in a ready-to-use ex vivo culture system.
Genoskin was founded in 2011, as a spin-off of the French National Center for Scientific Research (CNRS) and The Paul Sabatier University in Toulouse.
HypoSkin®, a patented injectable ex vivo human skin model that maintains the immunocompetence and structure of in vivo human skin.
Media contact:
Lorna Santos
Vice President of Marketing and Product Management
Genoskin
Follow us on Linkedin!
Comments are closed.