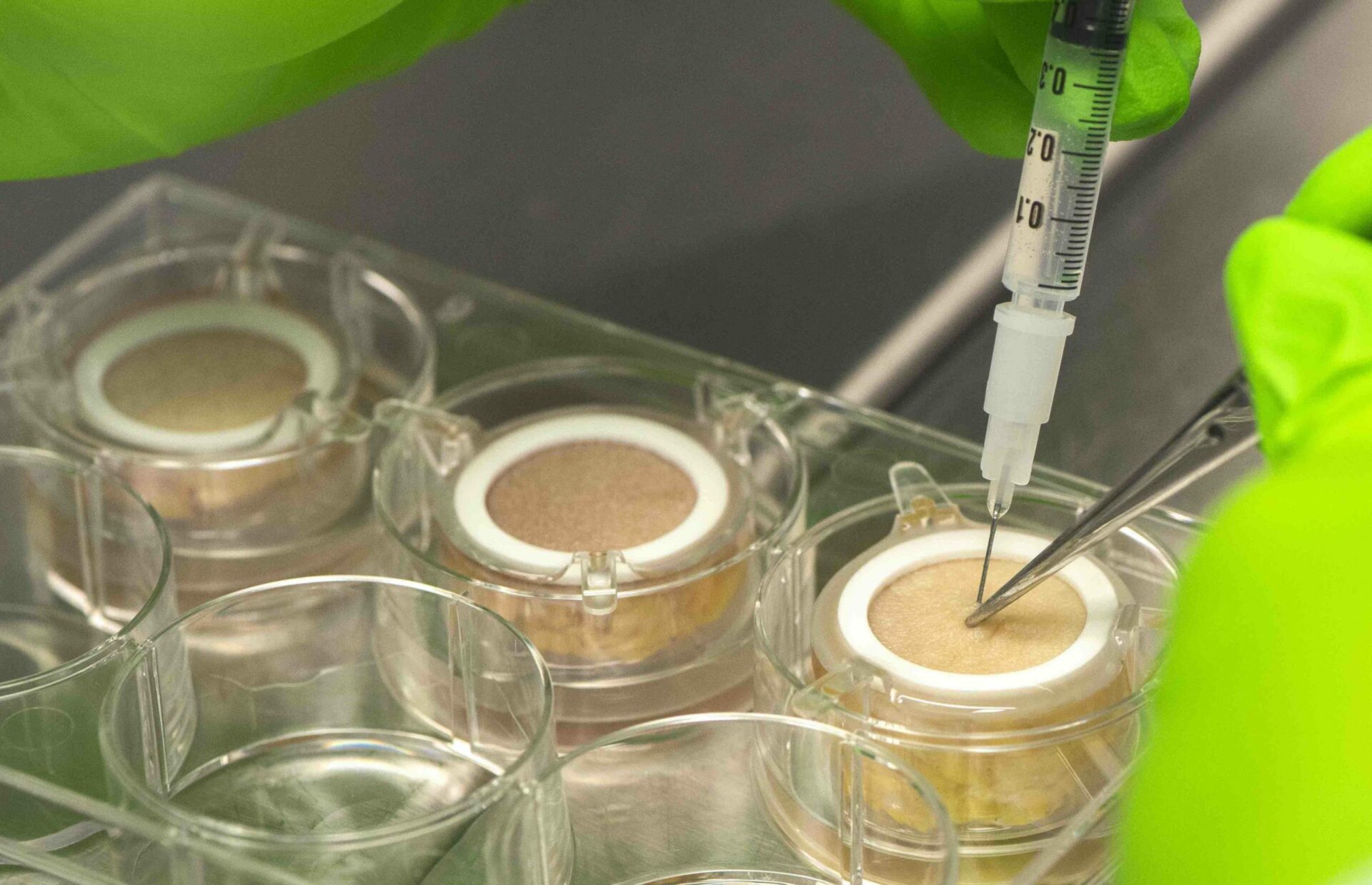
Genoskin granted a new patent on ex vivo subcutaneous injection model
The French National Institute for Intellectual Property (INPI) granted Genoskin’s latest patent on its unique ex vivo subcutaneous injection model, HypoSkin®.
A boom in the subcutaneous biologics market
Today, subcutaneous injection is one of the most common methods to deliver a drug into the human body. Its use has increased significantly since 2017 with an impressive annual growth rate of 7%. Over the past 10 years, the FDA approval of biopharmaceuticals administered by subcutaneous injection has increased by 333%. It is forecasted that the subcutaneously delivered drug market should reach $171 bn by 2025.
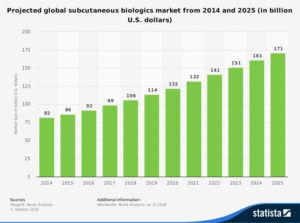
Projected growth of subcutaneously delivered biologics. Source: Statista 2018
This growth is due in part to the increase in the effectiveness of biopharmaceutical and biosimilar drugs. Their subcutaneous administration appears to be the safest and most cost-effective way to deliver them in patients. The utility of viscous biologics in the treatment of chronic diseases like diabetes and the requirement of a high concentration of subcutaneous formulation of biologics are also driving factors behind the market evolution.
In addition, the increasing trend of self-medication has pushed medical devices companies to innovate on wearable injectors, autoinjectors, pen injectors, needle-free injectors, prefilled syringes, drug reconstitution systems, and implants.
Another growth factor is the displacement of intravenously injected drugs by subcutaneously delivered biologics. Intravenous injections require a clinical setting for dosing and higher costs in development and treatment. On the opposite, subcutaneous injections allow reduced time spent in drug administration, reduced patient time at healthcare facilities and better therapy compliance. Reformulation of such therapeutics leads to reduced costs for pharmaceutical companies, healthcare facilities, and patients.
Testing new formulations: the need for a reliable in vitro model
The development of new drug formulations generally implies preclinical tests in vivo on animals (mice, rats, dogs, pigs, etc.) even though their skin and subcutaneous fat tissue can be drastically different from humans. As a result, no animal models are recognized as universal and reliable to predict human subcutaneous availability. The low predictability of animal models used in preclinical tests leads to 88% of drug attrition during clinical trials. This results in an estimated $1B additional cost in development per drug. Animal experiments also present strong limitations regarding ethical issues.
Therefore, pharmaceutical and medical device companies need a highly predictive and reliable in vitro model. This would enable them to analyze and predict the local human biological response such as injection site reaction and bolus analysis, injected drug distribution and absorption, etc. Effective testing to validate their new subcutaneous injection technologies will allow them to develop new drugs and medical devices cheaper and faster.
HypoSkin®, the first human ex vivo model for subcutaneous injections
Aiming to meet pharmaceutical companies’ needs, Genoskin developed HypoSkin®, the first human ex vivo model for subcutaneous injection. HypoSkin® is a unique, highly predictive and ethical human model that replaces animal testing. It contains living ex vivo human skin biopsies with attached subcutaneous adipose tissue that can be used to reliably test subcutaneous injections of drugs.
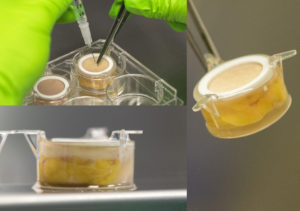
HypoSkin®, the first human ex vivo model for subcutaneous injections
Genoskin core technology consists in the use of an innovative and patented nourishing liquid matrix that becomes solid when excised human skin biopsies are deposed at the waterline. Hypodermis and dermis are then completely embedded in the solidified matrix while the epidermal surface is still in contact with air. The matrix enables normal cell viability and 3D architecture of subcutaneous tissue for 7+ days. Researchers can then perform the preclinical testing needed for the development of subcutaneous injection drugs & devices. Examples are compound absorption, first-pass catabolism, local toxicity, device/skin interactions.
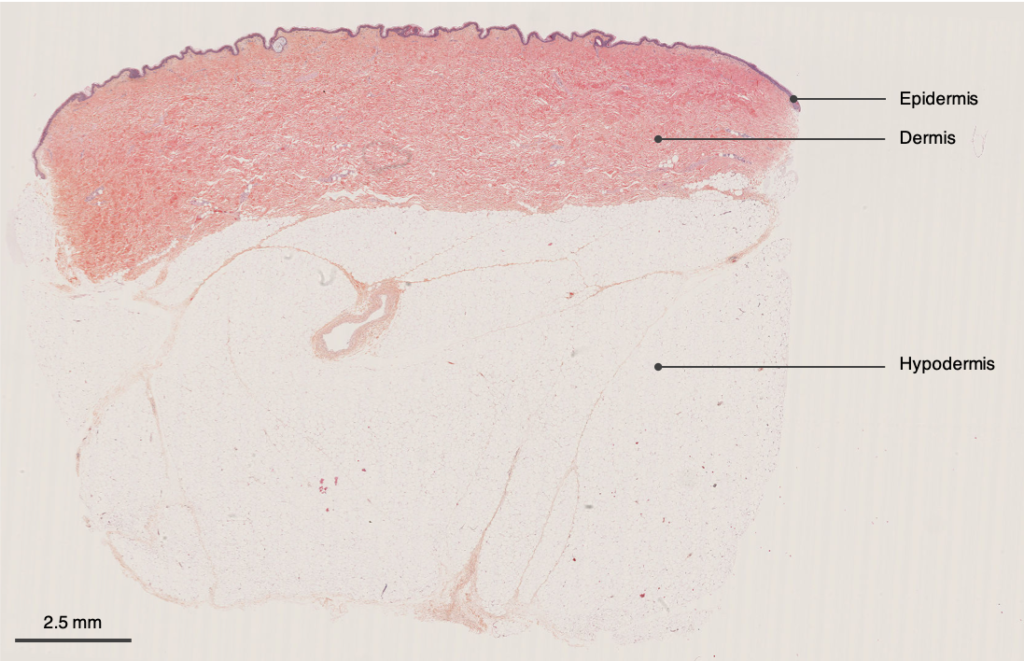
HypoSkin® assay contains all three skin layers: epidermis, dermis, and hypodermis
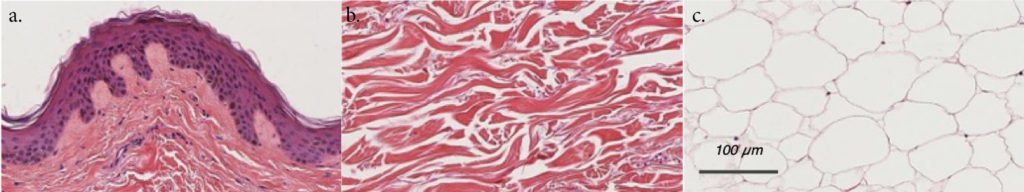
H&E pictures of the three layers of HypoSkin®. a. Epidermis, b. Dermis, c. Hypodermis
Reliably predict with HypoSkin® the local human’s response to subcutaneous injection during preclinical trials. Genoskin customers save time (10X faster than animal experiment), money (5X cheaper than animal experimentation) as well as secure the development of their new products before clinical trials.
To learn more about HypoSkin® technology, feel free to take a look at the following posters:
- Evaluation of local inflammatory reactions following subcutaneous injection of a pro-inflammatory cocktail in a fully human ex vivo skin model
- A fully functional ex vivo human skin model to study human skin microbiome
-
An ex vivo human skin model for the healing of infected wounds
FOR MORE DETAILS ON HYPOSKIN® AND ITS USE TO PREDICT TOXICITY AND EFFICACY OF INJECTABLE COMPOUNDS
Request our User Manual
To keep up-to-date with Genoskin’s latest news, follow us on Twitter and LinkedIn. You can also contact us to learn more about our products and services.
Comments are closed.