CREATING BETTER TECHNOLOGY FOR RELIABLE DRUG DEVELOPMENT STUDIES
Changing drug development as we know it
Genoskin aims to change drug development technology as we know it. We combine artificial intelligence and cutting-edge technology with live ex vivo human tissue to efficiently help pharmaceutical and biotech companies develop new drugs and vaccines in a better, faster and more cost-efficient way. And the cosmetic industry also benefits from our approach. Here’s why and how we do it.
The need for better drug development technology
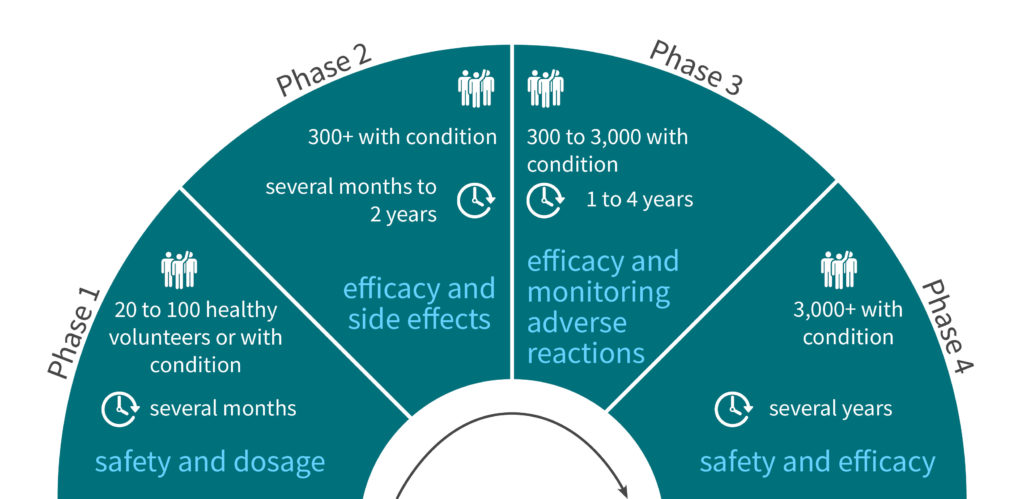
9 out of 10 drug development projects will fail

Clinical development success rates for investigational drugs remain a difficult issue. Despite various initiatives and efforts to improve success rates in both the public and private sectors, the likelihood to obtain an FDA drug approval from phase I clinical trials remains at ~10%. To make this pill even harder to swallow, the average cost associated with developing a drug to the approval stage has increased since the 2000s, reaching US $2.6 billion in the first half of the 2010s, according to a report by the Pharmaceutical Research and Manufacturers of America (PhRMA). This situation is hardly acceptable, as only 10% of drug development projects actually end up on the market to effectively treat patients or help them manage their condition and symptoms.
The poor predictability of animal models
One reason is the poor predictability of animal models. Before proceeding to clinical trials on humans, today’s scientists validate drugs in animals using a range of accepted processes and procedures to limit risks. Since the 1938 Food, Drug & Cosmetic Act, animal testing has been a regulatory requirement to obtain FDA approval before putting new drugs on the market in the US. This approach does not only raise ethical questions, animals are biologically different from humans. Whatever the test results may be on animal models, they rarely reflect what will happen when drugs are tested on humans during clinical trials.
The FDA Modernisation Act of 2021

The recently submitted FDA Modernisation Act of 2021 aims to allow drug manufacturers and sponsors to use alternative testing methods to investigate drug safety and effectiveness. Besides animal tests, the FDA Modernisation Act of 2021 would also allow innovative test methods such as cell-based assays, organ chips and microphysiological systems, sophisticated computer modelling and other human biology-based test methods, such as Genoskin’s live ex vivo human skin models.
New technology to obtain better human data for drug development
Using skin leftovers from plastic surgery for real human skin models

Genoskin uses a unique approach to generate real human data on new drugs in a better and faster way. Every year, hospitals and clinics across the world destroy well over 700,000 skin leftovers from plastic surgery procedures, such as tummy-tucks. This tissue is actually perfect to develop R&D study tools for human drugs that require topical or systemic administration. The solution is not new as such. A number of companies, such as biobanks, provide discarded human skin tissue to biotech, pharmaceutical and cosmetic companies for testing purposes, but they often freeze the obtained tissue before sending it to customers. However, freezing skin tissue will kill cell activity, which limits its use and the data researchers can obtain.
Improving the reliability of artificial skin model technologies
To bypass this problem, a lot of companies are spending millions to develop in vitro models, artificial skin tissue, human skin equivalents, bioprinted skin, reconstructed skin and organs-on-a-chip in order to mimic human organs and skin tissue. The goal of all these models is to obtain an as-close-to-human-as-possible response from the “organ” or “skin tissue” to the drug that is under development. However, all these technologies remain artificial in the sense that they are lab-made and do not contain all the cells, appendages and response types you would obtain from real human skin. They are definitely a step in the right direction but still have a lot of limitations when it comes to obtaining real human response during drug development.
The technology to keep tissue alive for real, live human data
The novelty with Genoskin’s models is that we have the technology to keep skin leftovers alive. We use a nourishing gel to keep skin biopsies alive, functional and, even more importantly, immunocompetent for up to 7 days, which allows for a full week of drug or cosmetic testing. Not only does our technology help you obtain real human response to compounds applied on and in the skin through topical, systemic or subcutaneous administration, it also allows for live human response without harming either humans or animals. All our skin models are sourced in full compliance with ethical and regulatory standards and standardized to allow for reproducible results. All our production processes are ISO9001 certified.
Combining live human data with cutting-edge technology for reliable results
Genoskin’s unique technology allows researchers to test drugs and compounds that require topical, systemic or subcutaneous administration on a range of data human data generation platforms. These platforms include healthy skin, inflamed skin, skin with psoriasis characteristics, wounded skin and even skin models for subcutaneous injection. The latter is a huge step forward: Genoskin’s HypoSkin® model is the first skin model in the world that allows testing injections on humans outside of humans. Genoskin’s Injection Site Reaction platform is designed around this skin model, to enable researchers to study injection site reactions and adverse events on and in human skin. Our R&D departments use cutting-edge technology for efficient and reliable data analysis, such as the MANTIS® Spatial Biology platform.
Small-scale clinical trials without humans
We’re even able to combine artificial intelligence with the natural variations in skin phototype, age and gender to organize small-scale clinical trials with real human data but without actually using humans. The variations in our skin models are donor-dependent and are one of the great strengths of our approach, as they reflect natural skin variations in the general population, allowing for more reliable and relevant study results.
Comments are closed.





