Alternatives to animal testing
Pilot studies – Local toxicity assesment
Request a toxicity pilot study plan and quote to evaluate local tolerance and immune effects in ex vivo human skin models.
APPLICATION NOTE – Toxicity services for pharma, biotech & medical devices developers
Download this application note and discover 3 case studies. Refine formulations or assess local tolerance in human skin before clinical trials.
WHITE PAPER – HypoSkin® & immunogenicity testing
Explore how HypoSkin®—a standardized, injectable, and immunocompetent human skin model—provides a translational, animal-free solution for assessing the immunogenicity of therapeutic compounds. From structural stability to real-time immune response profiling, this white paper delivers data-backed insights designed for pharma and biotech teams advancing injectable drug development.
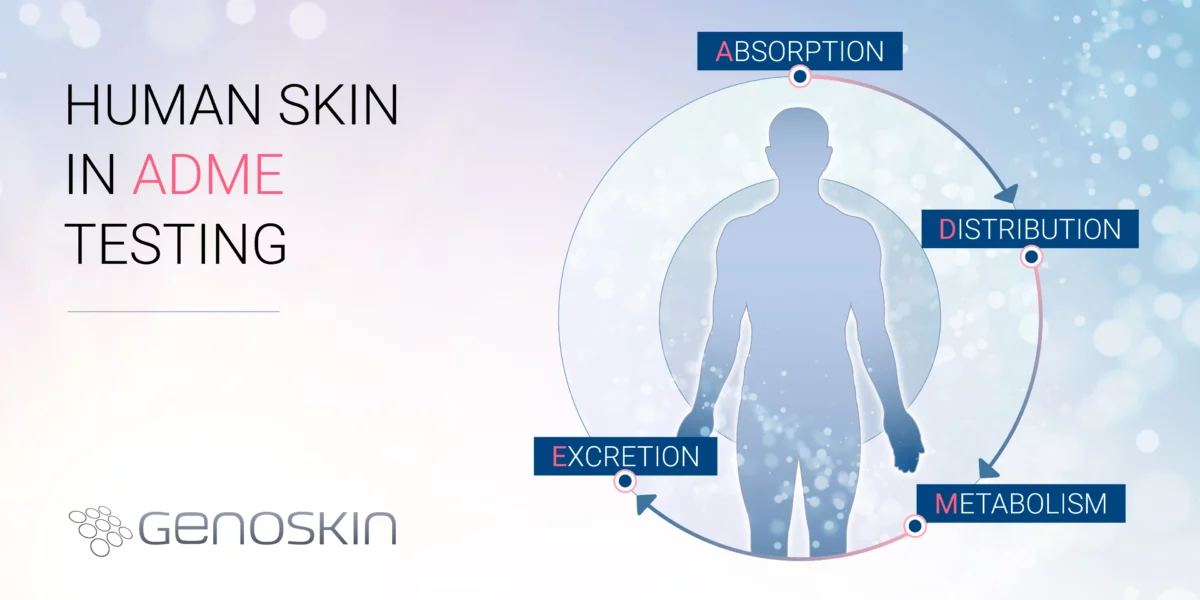
Beyond animal models: The role of human skin in ADME testing
From human skin to human data: Advancing immunogenicity testing with human models
APPLICATION NOTE – HypoSkin® services
HypoSkin services combine an ex vivo skin platform with new generation transcriptomic, cytokine analysis and bioinformatics to support your R&D with human insights.