News
Genoskin maintains US lab activity amid COVID-19 pandemic
COVID-19 update: Genoskin to maintain US production activity during pandemic. In light of the latest updates on the COVID-19 pandemic, Genoskin’s top priority remains both employees and collaborators’ safety. Thanks to the ability to rely on skin expertise and model production in the US location (Salem, MA), Genoskin continues to provide partners with the available support needed in reliably and quickly
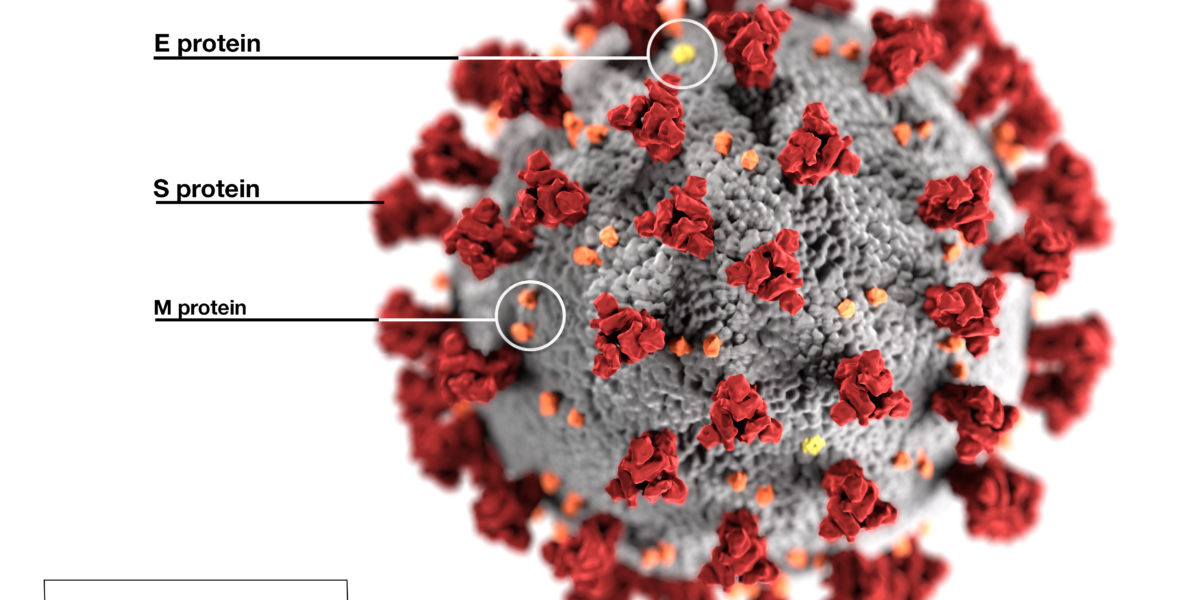
Genoskin supports CoViD19 research!
Genoskin COVID-19 research preferred access program On Friday, March 13th 2020, Genoskin launched a preferred access program for all research teams actively developing therapeutic and vaccine technologies for the COVID-19 pandemic. The World Health Organization (WHO) officially declared on March 11th, the outbreak of COVID-19 a pandemic. The disease caused by SARS-CoV-2 has spread globally. Over 130,000 individuals are affected so