Toxicity
10
Feb
From human skin to human data: Advancing immunogenicity testing with human models
From human skin to human data: Advancing immunogenicity testing with human modelsIn a public workshop organized by the FDA-CRCG group, Dr. Nicolas Gaudenzio, CSO of Genoskin, introduced an innovative platform for assessing immune reactions in response to injected substances using human skin explants. His talk, titled "A new in vitro framework for assessing immunogenicity using comprehensive profiling of injectable humanRead more
8
Oct
POSTER – Injection local inflammatory reaction IID2018
Scientific posterEvaluation of local inflammatory reactions following subcutaneous injection of a pro-inflammatory cocktail in a fully human ex vivo skin model. Poster IID2018.Subcutaneous (SC) injections can evoke local reactions such as inflammation or necrosis. Preclinical development of SC therapies relies on animal models to characterize these responses, however, translation of these models to the clinic is often limited and no singleRead more
22
Jul
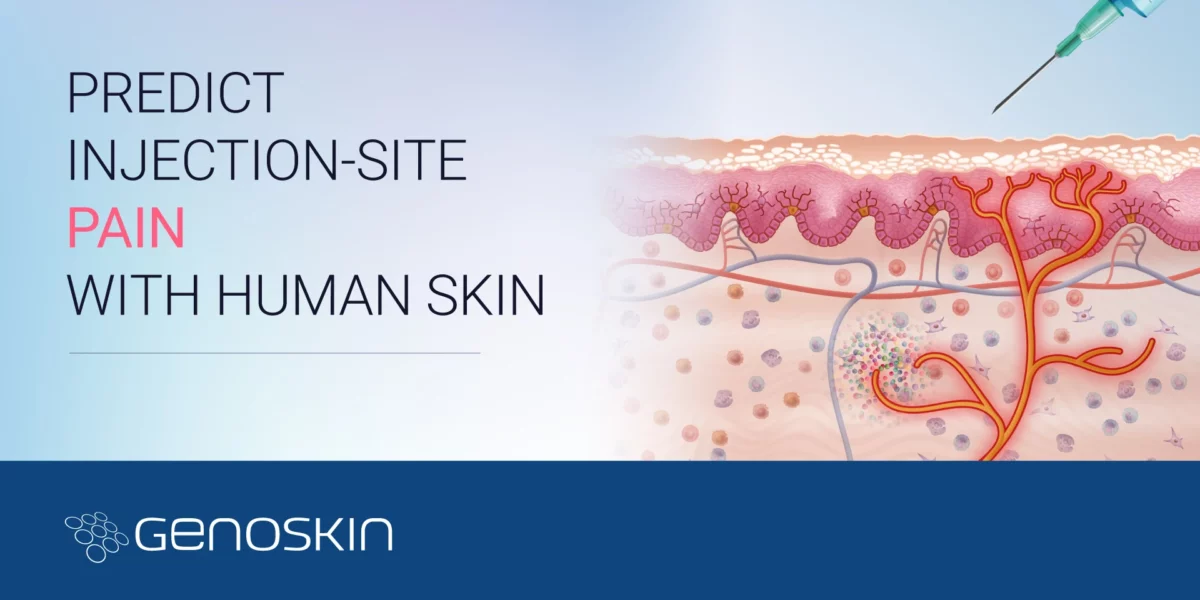
Injection-Site Pain
Injection-Site PainWhich factors influence pain upon subcutaneous injection?Biologics such as vaccines, insulin, monoclonal antibodies, etc., that are now widely developed need to be administered parenterally. While the intravenous (IV) route has historically been the most widely used, the subcutaneous (SC) route is now accepted and presented as safe and effective, whether in the context of chronic disease or in palliativeRead more
26
Jun
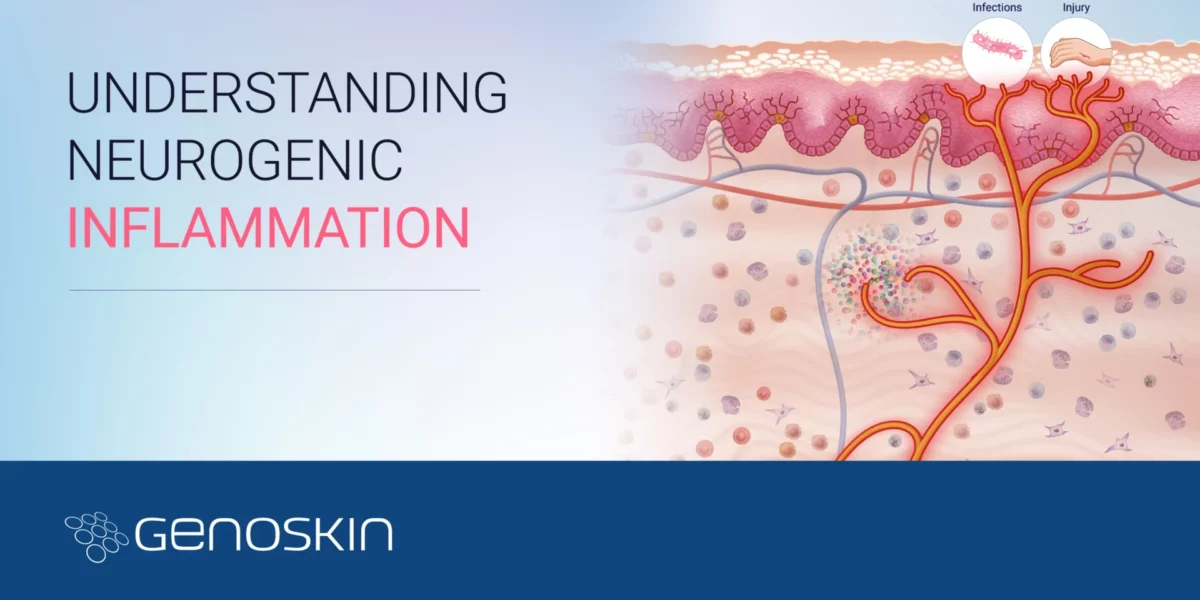
Understanding neurogenic inflammation
Understanding Pain & Neurogenic InflammationCan our understanding of pain and neurogenic inflammation help in predicting and managing injection-related pain?Nociception, the neural processes of encoding and processing noxious stimuli, and the sensation of pain are initiated by extreme pressures, temperatures, toxic substances, and inflammatory mediators capable of causing tissue injury. These strong stimuli are recognized by specific nerve cells in theRead more
12
Jun
CASE STUDY – Cetrorelix & ISR
Discover how the ImmunoSafe: ISR Platform® support the evaluation of local immune reactions caused by subcutaneous administration of Cetrorelix.
12
Jun
APPLICATION NOTE – ISR
Explore how the ISR Platform® can effectively de-risk your injectable therapeutic candidates today!