Immunogenicity Series
Part 4 – Evaluating and managing immunogenicity for vaccines
Immunogenicity is crucial in vaccine development, it represents the capacity of a substance, such as a vaccine, to elicit an immune response.
This reaction can be beneficial, providing protection against diseases, or detrimental, resulting in adverse effects. In vaccine development, the goal is to trigger on-target immunogenicity to activate the immune system without inducing the disease itself. Assessing and managing vaccine immunogenicity then becomes a critical issue as it will determine its ability to provoke a robust and protective immune response. Effective management of immunogenicity is essential for ensuring the safety and efficacy of vaccines, thereby serving as a fundamental element in the vaccine development process.
Optimizing Vaccine Immunogenicity
Optimizing the immune response to vaccines is fundamental for their effectiveness and safety. The use of adjuvants and the development of precise formulation strategies are critical to developing vaccines that elicit strong immune responses while maintaining safety.
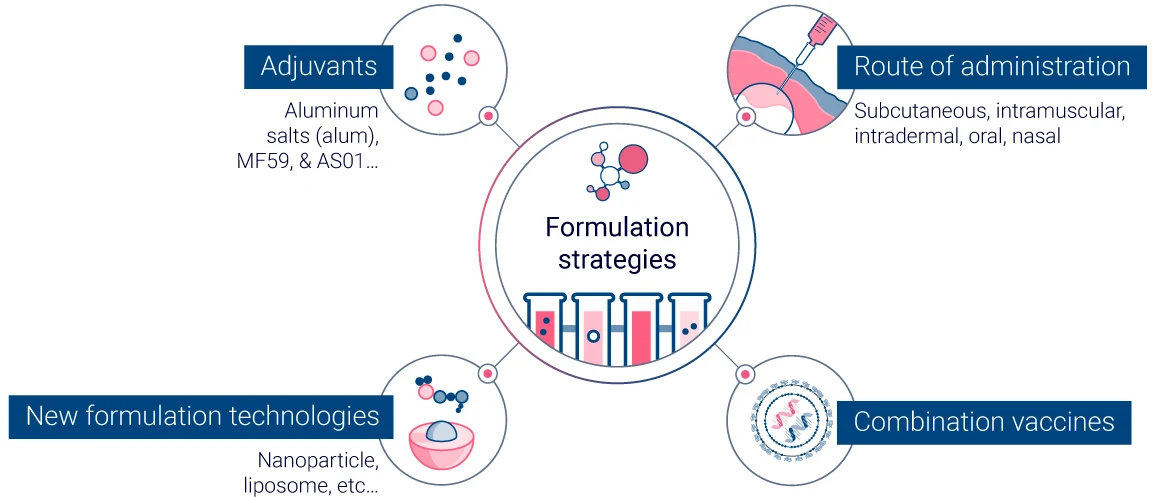
Adjuvants and Formulation Strategies
To enhance the immune response, vaccine developers frequently incorporate adjuvants, which help trigger a more robust reaction to the vaccine. Adjuvants, such as aluminum salts (alum), MF59, and AS01, are designed to enhance the body’s immune response to the antigen, promoting a stronger and more prolonged immune reaction. They work by creating a localized inflammatory response at the injection site, recruiting immune cells, and facilitating the presentation of the antigen to these cells. This process not only increases the production of antibodies but also helps in generating a robust T-cell response, which is essential for long-term immunity.
Formulation strategies also play a critical role in managing immunogenicity. The route of administration, whether intravenous, subcutaneous, intramuscular, or oral, can significantly influence the immune response. For example, intramuscular and subcutaneous injections are commonly used for their ability to deliver vaccines into tissues rich in immune cells, enhancing antigen uptake and presentation. Oral vaccines, although more convenient, must be formulated to withstand the harsh gastrointestinal environment to ensure that the antigen reaches the immune system intact.
Additionally, the use of nanoparticle and liposome technologies in vaccine formulation has shown promise in enhancing immunogenicity. These delivery systems can protect the antigen from degradation, target it to specific immune cells, and release it in a controlled manner to ensure a sustained immune response. The stability and release profile of the vaccine can be further optimized by modifying the physical and chemical properties of the formulation, such as particle size, surface charge, and encapsulation efficiency.
Furthermore, combination vaccines, which include multiple antigens from different pathogens, can be formulated to reduce the number of injections required and to enhance overall immunogenicity through the synergistic effect of the included adjuvants and antigens. These strategies not only improve the immune response but also enhance vaccine compliance and coverage rates.
Balancing Safety and Efficacy
One of the major challenges in vaccine development is balancing immunogenicity with safety. For live vaccines, such as those against measles or rotavirus, the goal is to achieve strong immune activation without causing the disease itself. These vaccines must replicate enough to provoke a robust immune response, yet be attenuated sufficiently to avoid inducing significant symptoms or complications resulting from the diseases. This delicate balance ensures that the immune system is adequately stimulated to develop long-term protection without posing unnecessary risks to the recipient.
Inactivated vaccines, often combined with adjuvants, aim to improve immunogenicity while maintaining safety. Adjuvants are carefully selected and formulated to boost the immune response to the antigen without introducing excessive reactogenicity. The challenge lies in identifying the right adjuvant and optimizing its concentration to enhance the vaccine’s effectiveness without increasing the likelihood of adverse reactions. Moreover, the formulation of inactevated vaccines involves meticulous attention to factors such as antigen stability, delivery method, and dosing schedules, all of which can influence the vaccine’s immunogenicity and safety profile.
Vaccine development strives for the delicate balance of maximizing protection against disease while minimizing the risk of severe side effects.
To enhance immunogenicity, vaccine developers may use adjuvants, which are substances added to vaccines to boost the immune response, or they may employ advanced vaccine technologies to mimic the natural infection process more closely. Overall, the ability of a vaccine to elicit a strong and effective immune response is central to its success in preventing and controlling infectious diseases.
Evaluation of On-Target Immunogenicity
Developing safe and effective vaccines involves comprehensive monitoring of biomarkers and immune responses to assess how well a vaccine stimulates the body’s defense mechanisms. Equally important is the rigorous testing through clinical trials, which are designed to evaluate the vaccine’s efficacy and safety in diverse populations. This detailed approach not only aids in optimizing vaccine formulations but also ensures that they provide long-term protection against infectious diseases.
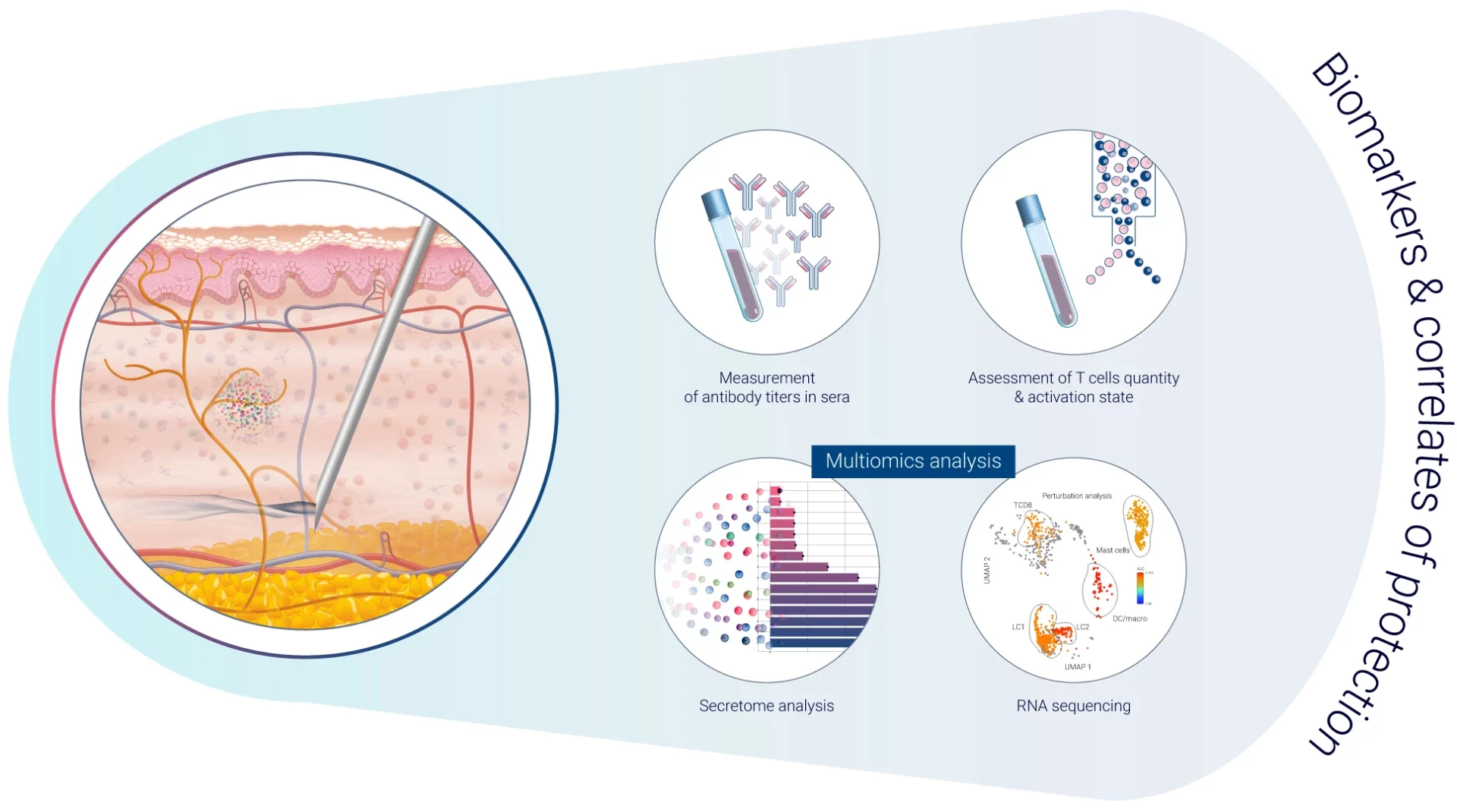
Biomarkers and Immune Response Monitoring
Biomarkers and immune response monitoring are integral components of vaccine development, providing crucial insights into the efficacy and safety of vaccines. Biomarkers, which are measurable indicators of biological processes or responses, help researchers understand how the immune system reacts to a vaccine. Monitoring these biomarkers enables the identification of specific immune responses, such as the activation of T cells or the production of antibodies, that correlate with protection against the disease1. This method not only aids in determining the optimal dosage and formulation of the vaccine but also assists in predicting long-term immunity and potential side effects. Through detailed immune response monitoring, scientists can refine vaccine designs to improve their effectiveness, ensuring a balanced immune response that provides long-lasting protection while minimizing side effects.
Vaccines provide effective protection against infectious diseases due to a combination of factors. One of the primary correlates of protection (immune function that is responsible for protection) is the development of neutralizing antibodies, which can recognize and bind to the virus or bacteria and prevent it from infecting cells. Another important factor is the activation of T cells, which can help to clear infected cells and provide long-term immunity. In addition, the type and duration of the immune response generated by the vaccine can also play a role in determining the level of protection provided. Other factors that can impact vaccine efficacy include the age and health status of the individual receiving the vaccine, as well as the genetic variability of the virus or bacteria being targeted. By understanding these correlates of protection, researchers can better design and optimize vaccines to provide the greatest possible protection against infectious diseases.
Clinical Efficacy Trials
Clinical trials are rigorously designed to assess vaccine effectiveness by evaluating immune responses against specific clinical outcomes. These trials are essential for identifying immune responses that predict protection, which is crucial for the rapid development and distribution of vaccines. Through detailed analysis of biomarkers and other immunological indicators, clinical trials can determine whether a vaccine elicits a sufficient and appropriate immune response. This includes assessing the activation of B cells and T cells, the production of neutralizing antibodies, and the development of immunological memory, all of which are key to long-term protection.
Furthermore, trials often include diverse populations to ensure that the vaccine is effective across different age groups, genders, and health conditions. The data gathered from these trials provide comprehensive insights into the vaccine’s safety profile, dosage requirements, and potential side effects, ensuring that the vaccine can trigger sufficient immunological memory to protect against the actual pathogen without causing adverse reactions. This meticulous process builds public confidence in new vaccines, supporting their regulatory approval, and ultimately facilitating their deployment to control and eliminate infectious diseases.
Additionally, human challenge trials (HCTs), where vaccinated individuals are intentionally exposed to the pathogen in a controlled setting, offer a unique and accelerated way to determine a vaccine’s protective capabilities. HCTs are a powerful tool for vaccine development. They can directly demonstrate efficacy, determine correlates of protection, and improve understanding of both disease pathogenesis and the human immune response. This comprehensive approach enables faster assessment of the vaccine’s real-world effectiveness2. By carefully analyzing the data from these trials, researchers can ensure that vaccines are both safe and effective before they are approved for public use. Despite their advantages, HCTs raise significant ethical concerns. Infecting healthy individuals carries inherent risks, including the possibility of serious illness or adverse events.
To enhance immunogenicity, vaccine developers may use adjuvants, which are substances added to vaccines to boost the immune response, or they may employ advanced vaccine technologies to mimic the natural infection process more closely. Overall, the ability of a vaccine to elicit a strong and effective immune response is central to its success in preventing and controlling infectious diseases.
Concluding Thoughts
This exploration of immunogenicity testing and management in vaccines highlights the intricate balance between triggering effective immune responses and ensuring safety and efficacy. The ongoing advancements in vaccine technology and our growing understanding of the immune system continue to play pivotal roles in developing safe and effective vaccines. By leveraging these advancements, researchers can design vaccines that elicit strong, protective immune responses while minimizing risks, ultimately leading to better public health outcomes and increased confidence in vaccination programs.
References
1Stanley A. Plotkin, Correlates of Vaccine-Induced Immunity, Clinical Infectious Diseases, Volume 47, Issue 3, 1 August 2008, Pages 401–409, https://doi.org/10.1086/589862
2Nguyen LC, Bakerlee CW, McKelvey TG, Rose SM, Norman AJ, Joseph N, Manheim D, McLaren MR, Jiang S, Barnes CF, Kinniment M, Foster D, Darton TC, Morrison J. Evaluating Use Cases for Human Challenge Trials in Accelerating SARS-CoV-2 Vaccine Development. Clin Infect Dis. 2021 Feb 16;72(4):710-715. doi: 10.1093/cid/ciaa935. PMID: 32628748; PMCID: PMC7454474.
Comments are closed.





