From human skin to human data: Advancing immunogenicity testing with human models
In a public workshop organized by the FDA-CRCG group, Dr. Nicolas Gaudenzio, CSO of Genoskin, introduced an innovative platform for assessing immune reactions in response to injected substances using human skin explants.
His talk, titled “A new in vitro framework for assessing immunogenicity using comprehensive profiling of injectable human skin modules,” sheds light on a transformative approach to immunogenicity testing. Here’s an in-depth look at the key highlights from his presentation.
The skin: a critical immune organ
The human skin is far more than a protective barrier—it is a dynamic immune ecosystem. With approximately 15 distinct subpopulations of immune cells, the skin plays a central role in orchestrating complex responses to foreign substances. For therapeutics designed for injection or implantation into the skin—representing roughly 23% of molecules in the development pipeline, according to the WHO Observatory—understanding these interactions is crucial for mitigating immunogenicity risks.
Genoskin’s human skin model: the HypoSkin® advantage
Genoskin has pioneered a platform using full-thickness human skin explants derived from surgical biopsies. Thanks to a patented technology, these skin models, composed of the epidermis, dermis, and hypodermis, retain their tissue architecture and cell viability, making them ideal for injection studies. They offer more translational relevance than traditional animal models from an immunological standpoint, offering greater relevance to clinical outcomes upon injection of therapeutic compounds.
The model is encased in a patented gel-like matrix that maintains the mechanical properties of the tissue and prevents the cells, particularly immune cells, from diffusing out of the model. A silicone right on top of the epidermis ensures optimal skin tension and contributes to the maintenance of the skin’s bio-mechanical properties. This is critical in preventing human skin from becoming too soft and lax after a couple of days. Together, this system preserves the viability and anatomical distribution of immune cells within the biopsy over time, even when injected with a therapeutic substance.
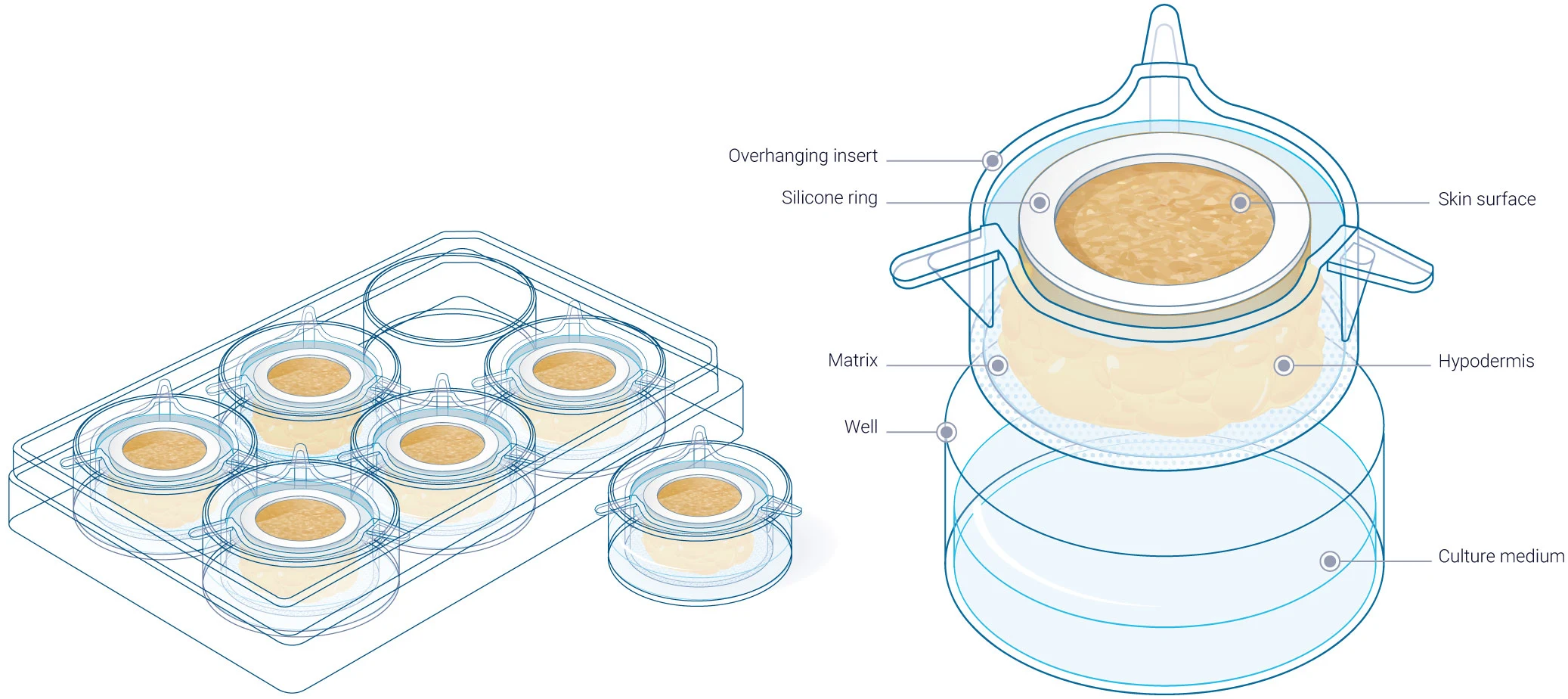
The HypoSkin® technology allows researchers to:
- Test various injection routes, including subcutaneous, intradermal, and transdermal delivery
- Observe immune responses, whether immediate or delayed by a few days
Additionally, it maintains stable gene expression profiles for up to 10 days.
Validating & applying the model
The HypoSkin system has been rigorously validated, ensuring accurate replication of immune dynamics. With the ability to assess responses across multiple donors and replicates, the platform guarantees robust statistical reliability. It has been extensively used to investigate immune responses to various drug candidates, reinforcing its reliability for preclinical testing.
One powerful application of this model is in unwanted immunogenicity risk assessment. For this purpose, the HypoSkin® model is integrated into the ImmunoSafe: ISR Platform®, which includes readouts such as cytokine release analysis and bulk or single-cell RNA sequencing. This platform supports the assessment of immune-related adverse events both before and during early-phase clinical trials.
Case study: assessing the Moderna COVID vaccine
Dr. Gaudenzio presented a compelling study using HypoSkin® to evaluate the immunogenicity of the mRNA-1273 COVID-19 vaccine. This experiment analyzed immune responses at both the tissue and cellular levels:
- Tissue-Level Analysis: Bulk RNA sequencing was employed to examine the entire skin ecosystem. This approach captured the broader effects of the vaccine on the structural cells and resident immune cells.
- Cell-Level Analysis: Single-cell RNA sequencing was used to closely examine specific immune cells involved in the vaccine uptake. This analysis provided critical insights into the dynamics of mast cells, dendritic cells, macrophages, and other immune cell subsets affected by vaccine treatment.
These techniques provided an unparalleled understanding of how the vaccine interacts with and influences the skin’s immune landscape.
The data were published in the Allergy, the official journal of the European Academy of Allergy and Clinical immunology, in December 2024 under the title “Multimodal profiling of biostabilized human skin modules reveals a coordinated ecosystem response to injected mRNA-1273 COVID-19 vaccine.”
Understanding the skin ecosystem
The ability to assess expected immunogenicity, as demonstrated in our study with the mRNA-1273 COVID-19 vaccine, highlights the broader potential of our platform. The ImmunoSafe: ISR Platform®, powered by HypoSkin®, can also be leveraged to evaluate unwanted immune responses to other biologics, including injection-site reactions and immune-related adverse events.
By identifying key immune cell subsets—such as dendritic cells and mast cells—that drive these responses, our platform provides critical insights into therapeutic safety and immunogenicity. This approach helps optimize biologic formulations to minimize adverse effects while maintaining efficacy
A new era in immunogenicity testing
Dr. Gaudenzio’s presentation underscored a paradigm shift in preclinical and non-clinical immunotoxicity assessment. By preserving the intricate interactions of human skin, Genoskin’s platform equips researchers with a detailed, physiologically relevant framework to de-risk injectable therapeutics.
As advancements like these continue to redefine drug development, human-centered alternative methods such as HypoSkin® are leading the way for safer and more effective therapeutics.

Watch the recorded talk
Day 2, Session 4, from 1:49:00 to 2:08:00.
Comments are closed.