Human Peripheral Blood-derived Cultured Mast Cells
A cellular model to study injection site reactions & severe allergic reactions.
In vitro mast cell culture assays are critical to better understand the kinetics and dynamics underlying inflammatory reactions.
Genoskin has developed a method to culture Human Peripheral Blood-derived Cultured Mast Cells (PBCMCs) from healthy individuals.
We isolate CD34+ progenitors from PBMCs of donors using specific culture and maturation media and obtain mature, ready-to-use human PBCMCs after a 6 week process.
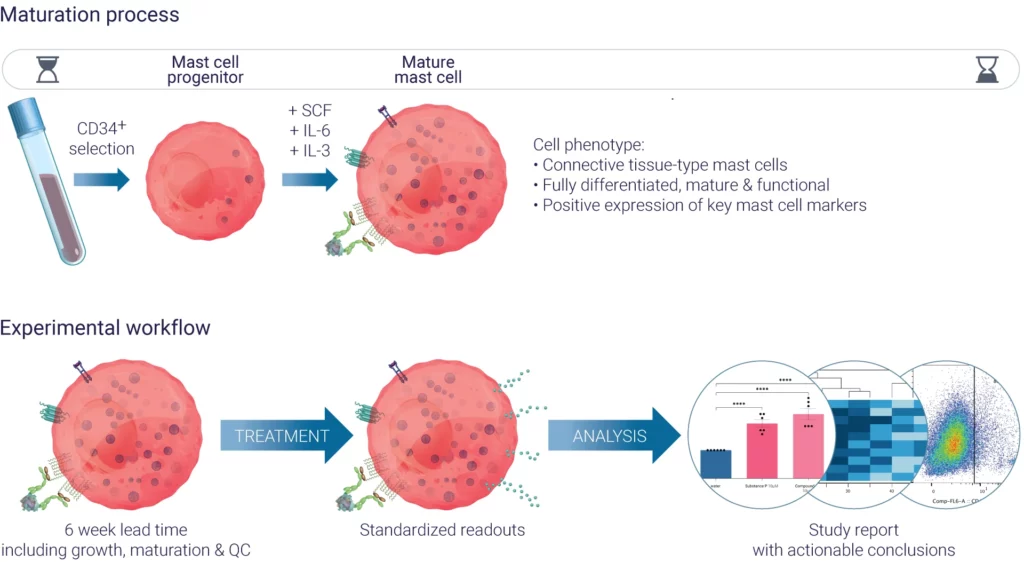
Figure 1: Outline of maturation process and experimental workflows for using in vitro cultured PBCMCs.
In vitro cultured mast cells are essential for studying how specific compounds trigger degranulation and for uncovering the underlying mechanisms of mast cell activation. They also provide a valuable platform to quantify de novo secretion of mast cell-associated cytokines in the supernatant, enabling detailed analysis through multiplex cytokine assays.
What makes human primary mast cells indispensable for preclinical studies?
Mast cells are long-lived innate immune cells derived from both embryonic and hematopoietic progenitors that have migrated and completed their maturation in tissues, including the skin (Ref.1). They are located in connective tissues and mucosa, at the junction of a host and its external environment where they act like sentinel cells against pathogens.
Connective Tissue-type Mast Cells (CTMCs) are a type of mast cell that have been studied and identified in both murine rodents and humans (among other mammals). They are found in the peritoneal cavity, gastrointestinal tract, and skin. Their cytoplasm contains granules filled with heparin, proteoglycan, and high amounts of histamine. Mast cells are known to differ phenotypically distinct across mammalian species, with notable distinctions between mice and humans.
Fortunately, human mast cell assays can now be conducted at three distinct levels:
1) Macroscopic, using skin tissue,
2) Cellular, using innate mature mast cells; and
3) Molecular, focusing on receptor-specific responses.
Are we overlooking a critical pathway in drug safety testing? IgE isn’t the whole story.
Mast cells are known to play a key role in initiating acute allergic reactions such as anaphylaxis, a severe IgE-mediated hypersensitivity response triggered by harmless substances found in medicines and food. While IgE-driven reactions are commonly associated with mast cell activation, recent research reveals that mast cells are not limited to just this pathway.
In fact, mast cells can be activated by a wide array of receptors, each playing a unique role in immune responses. One particularly important receptor gaining attention is MRGPRX2 (Mas-related G-protein coupled receptorX 2; MRGPRB2 is the mouse ortholog) (Ref. 2), a mast cell-specific receptor found in human skin. This receptor is pivotal in mediating mast cell activation through non-IgE mechanisms, and can be activated by a large panel of cationic molecules such as neuropeptides (e.g., substance P), venom peptides, host defense peptides, and also a number of FDA-approved drugs.
Once bound to one of its ligands, MRGPRX2 induces mast cells to release cytokines, lipids, and granule-associated proteases which modulate immune cell recruitment, vascular permeability, and activates sensory nerves. This mechanism leads to symptoms such as severe itch (pruritus), pain, and local inflammation.
While not intended to target the immune system, some drugs can alter or trigger these immune reactions. Such mechanisms can be observed in injection site reactions also called ISR . While ISR frequency varies depending on active compounds and patients, it occurs in about 15 % of patients and can negatively affect patient adherence to treatment
Although immediate ISRs present common symptoms with a hypersensitive reaction, they are not IgE-mediated; instead, they are driven by MRGPRX2 activation, and can occur without previous sensitization to the trigger.
Recent studies have identified that many drugs that trigger ISR contain a THIQ motif (e.g.,. tetrahydroisoquinolinone) in their molecular structure. This characteristic is promising because it provides a reliable and precise tool for preclinical trials to either eliminate drug candidates with potential side effects or select them to target specific receptors, such as MRGPRX2, to treat certain allergic reactions.
Previous research has shown that cationic signals (e.g., via substance P), in contrast to canonical FcεR-mediated activation, can induce very different mast cell degranulation dynamics, leading to distinct inflammatory responses in vivo.
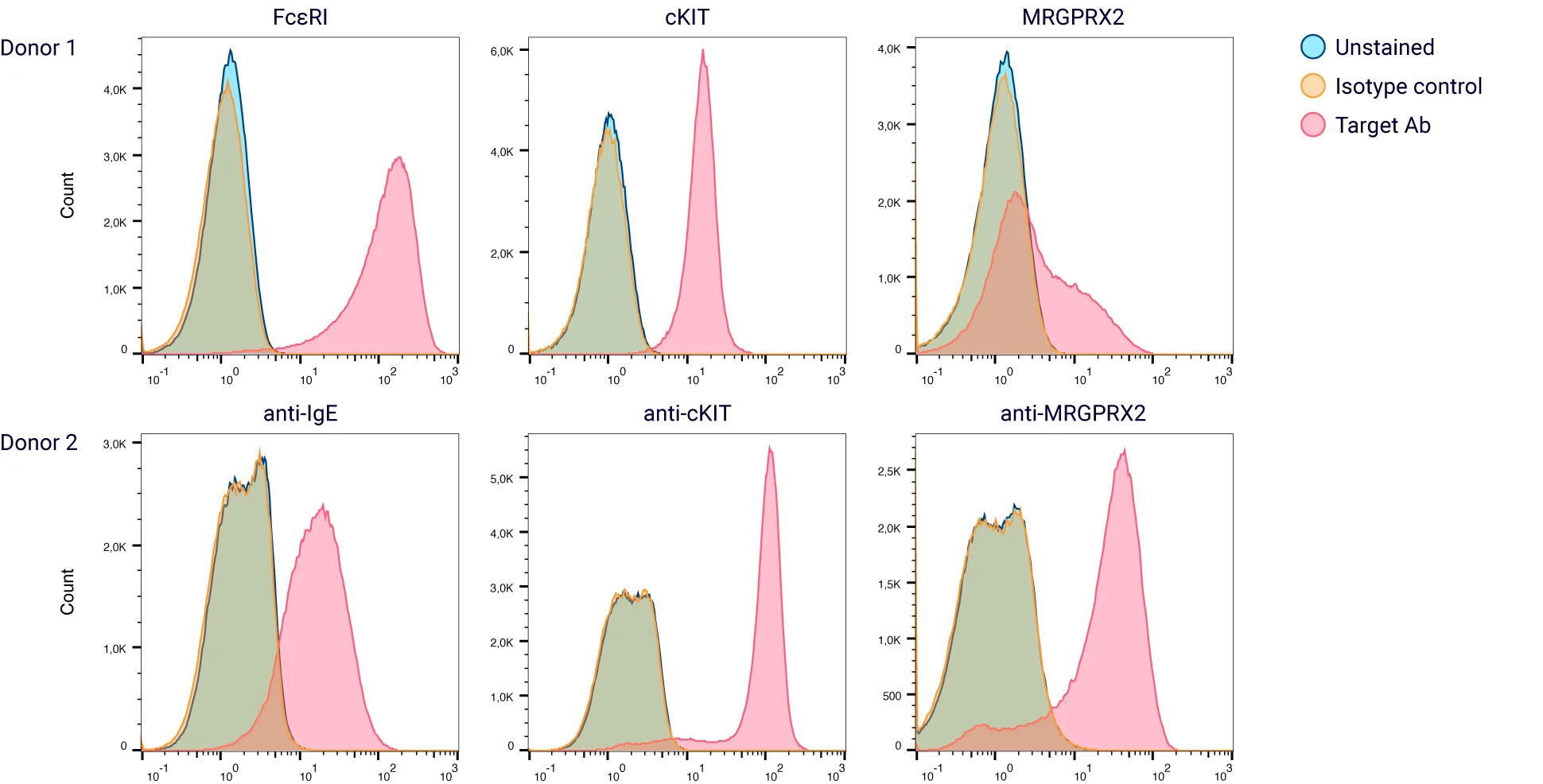
Figure 2: The majority of the in vitro differentiated HCPBMCs co-express cKIT and FcεR1α; the expression of MRGPRX2 is variable between donors.
Mast cells are essential in preclinical testing as they provide valuable insights into the type of inflammation that may occur in clinical trials and human tissue assays. By screening mast cells from a broad range of donors, we can better predict population-wide reactions to new biologics and strengthen the data for FDA approval.
Sources
- Annu. Rev. Immunol. 2020. 38:49–77 ;Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns Stephen J. Galli, Nicolas Gaudenzio, and Mindy Tsai
- http://dx.doi.org/10.1172/JCI85538;Different activation signals induce distinct mast cell degranulation strategies Nicolas Gaudenzio, Riccardo Sibilano, Thomas Marichal, Philipp Starkl, Laurent L. Reber, Nicolas Cenac, Benjamin D. McNeil, Xinzhong Dong, Joseph D. Hernandez, Ronit Sagi-Eisenberg, Ilan Hammel, Axel Roers, Salvatore Valitutti, Mindy Tsai, Eric Espinosa, and Stephen J. Galli
- MRGPRX2 sensing of cationic compounds—A bridge between nociception and skin diseases?Auriane Corbiere | Alexia Loste | Nicolas Gaudenzio
- Injection site reaction – when our mast cells get emotional – N. Gaudenzio – MassBio Insider Winter 2020 – To be published
Comments are closed.




