VaxSkin®
Human immunological insights
for vaccine candidate selection
VaxSkin®
Human immunological insights for vaccine candidate selection
Multi-modal immunoprofiling of skin response to vaccines using VaxSkin®

Dive deep into human immunology with VaxSkin®. VaxSkin® is designed to enable the examination of the early stages of the human immune responses to vaccines and adjuvants in their natural environment at the site of injection.
With precision at its core, VaxSkin® identifies key immune responses and delivers crucial data ahead of clinical trials. Powered by HypoSkin® technology, VaxSkin® combines bio-stabilized, immunocompetent ex vivo human skin with multiomics and computational molecular pathway analysis, providing insights into the early stages of human immune responses to vaccine injection. Advance your research and mitigate developmental risks by accurately selecting the most promising vaccine candidates.
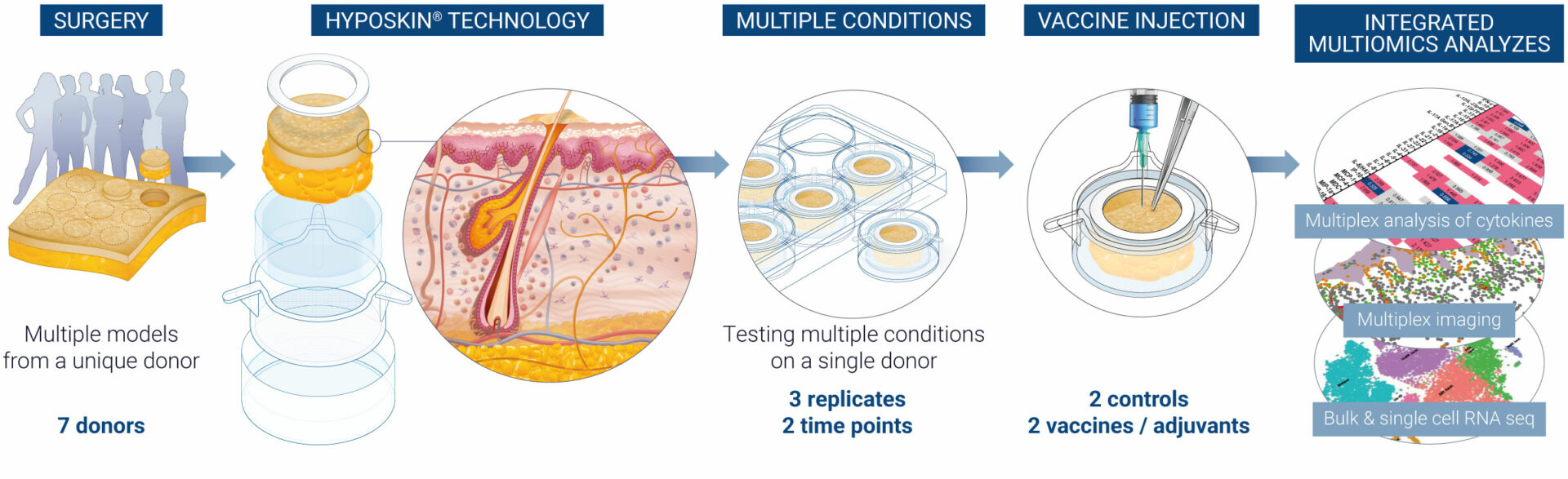
VaxSkin®: Integrated multiomic assays to select the most promising candidate

VaxSkin® is a platform built on four pivotal axes that allow a comprehensive immunoprofiling of the human skin response to vaccines over time at both the organ and single-cell levels.
ASSESS TISSUE-LEVEL INFLAMMATION
Assess the modulation of cytokines in response to vaccine injection. Uncover biological pathways activated upon vaccine injection based on cytokines secreted.
EVALUATE GLOBAL SKIN MODULATION UPON VACCINE INJECTION
Gain insights into the sequential interactions between the exogenous substance and the skin’s biological processes by uncovering the tissue-level gene expression pathways affected by the adjuvant and vaccine.
UNDERSTAND IMMUNE CELL TYPES INVOLVED IN VACCINE RESPONSE
Detect cell-specific transcriptomic modulations in multiple skin-resident cells after vaccination. Uncover which immune cells are involved in mRNA vaccine internalization and response.
ANALYZE APC ACTIVATION UPON VACCINE INJECTION
Investigate APC activation at the protein level with multiplex spatial imaging (MANTIS®).
Assess tissue-level inflammation
Assess tissue inflammatory response with multiplex cytokine release analysis.
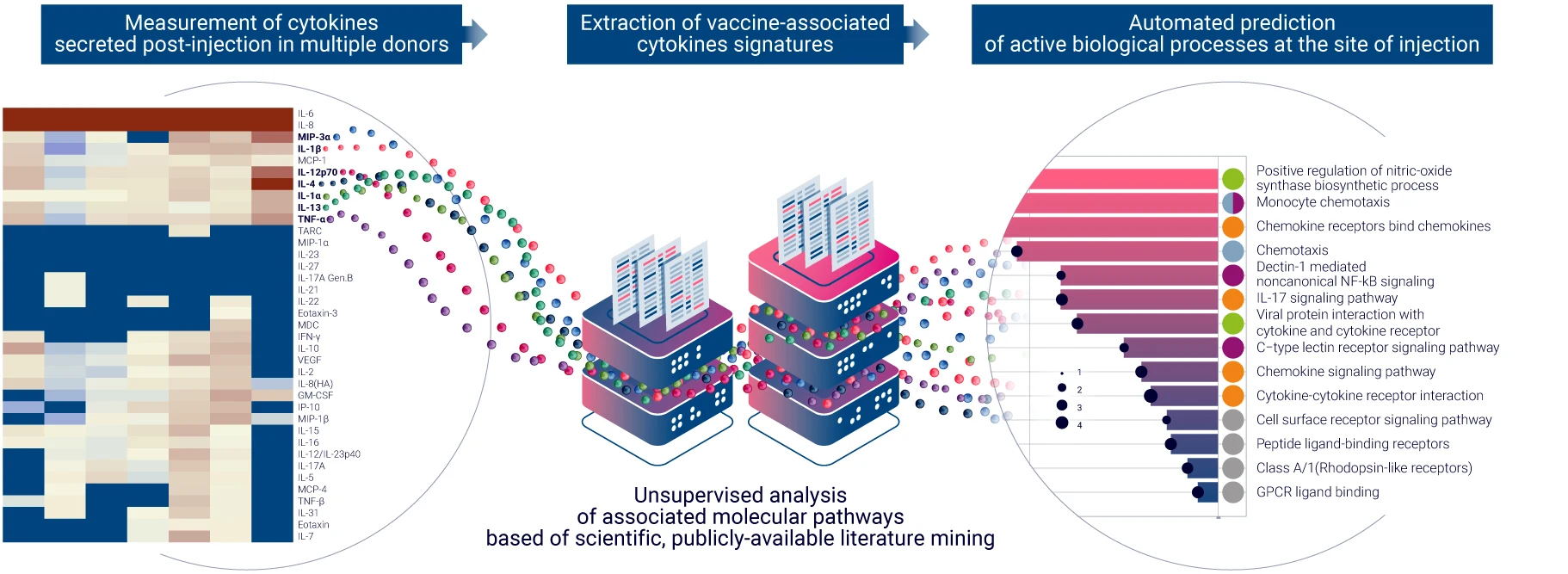
The ability of a vaccine to induce a global inflammatory response in the skin represents an early indication of its potency. With VaxSkin®, we employ multiplex cytokine release assays combined with a proprietary bioinformatics approach to differentiate cytokines directly influenced by vaccine injections from those exclusively modulated by adjuvants. VaxSkin® leverages AUDACY (Automated Data Analysis of Cytokines), our advanced bioinformatics-based analytics tool, to map the biological pathways associated with cytokine release that are triggered by vaccine administration. By pulling data from publicly available and peer-reviewed online databases, AUDACY provides a comprehensive view of the secretome and the immune mechanisms activated.
This tissue-level analysis offers valuable insights into a vaccine candidate’s ability to stimulate the secretion of cytokines and chemokines by activated antigen-presenting cells, helping assess their immunogenic potential with precision.
VaxSkin® ensures reliable results by using HypoSkin® models from many donors (10+). This method ensures statistically significant results of secretomic signature, allowing for more accurate characterization of the activated biological pathways implicated in the vaccine response.
Evaluate the global modulation of the tissue using bulk RNA sequencing
When a vaccine is administered, the entire skin ecosystem adapts to react to the external stimuli.
Using bulk RNA sequencing, subtle alterations in gene expression following vaccine administration can be detected.
This enables the distinction of the precise chain of events occurring between the exogenous substance and the skin’s biological processes.
VaxSkin® utilizes HypoSkin® models from multiple donors, which can be the same donors used for inflammatory response assessment.
After performing standard bulk RNA sequencing, VaxSkin® integrates our dedicated bioinformatics pipeline. We interrogate publicly available data to identify potential biological pathways associated with the detected differentially expressed genes (DEGs). We then provide comprehensive interpretation using our proprietary app FindYourPath. This offers crucial insights into specific biological mechanisms, such as immune responses and cell regulation, with the ability to generate clear, customizable graphical outputs for easy interpretation.
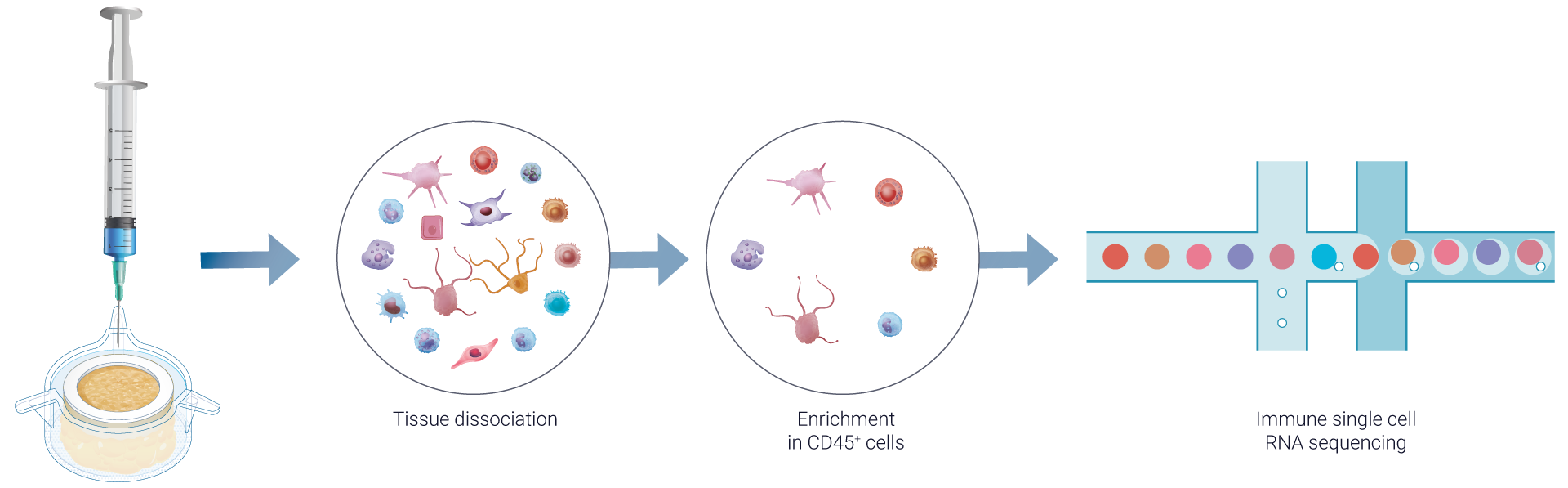
Understand which immune cell types are involved in vaccine response using single-cell RNA sequencing
VaxSkin® provides a detailed single-cell analysis, powered by single-cell RNA sequencing, to uncover cell-specific transcriptomic changes in various skin-resident immune cells following vaccination.
With VaxSkin®, we identify the biological pathways activated by the vaccine at the single-cell level, offering precise insights into innate immune mechanisms at the injection site.
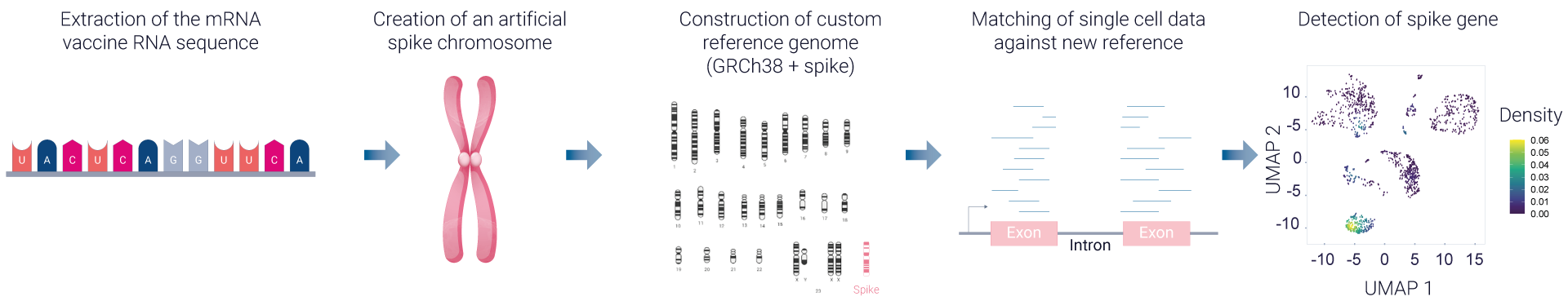
Additionally, VaxSkin® can track and quantify vaccine mRNA copies within cells, shedding light on related changes in transcriptomic profiles.
To deliver comprehensive immune profiling, we perform a single-cell RNA sequencing on dissociated HypoSkin® models post-vaccine administration, enriched in CD45+ skin-resident immune cells. This process, similar to the bulk RNA sequencing approach, integrates advanced bioinformatics and pathway analysis using FindYourPath, providing a deeper understanding of the biological functions influenced by vaccination.
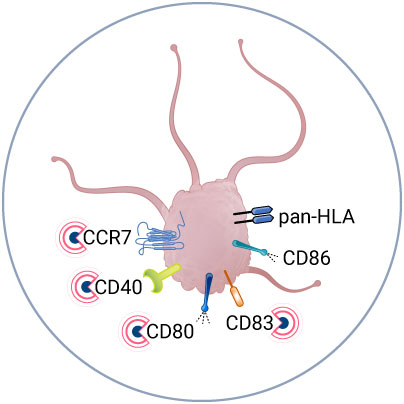
Analyze APC activation upon vaccine injection
For effective vaccination, it is crucial that antigen presenting cells (APCs) exhibit a mature phenotype.
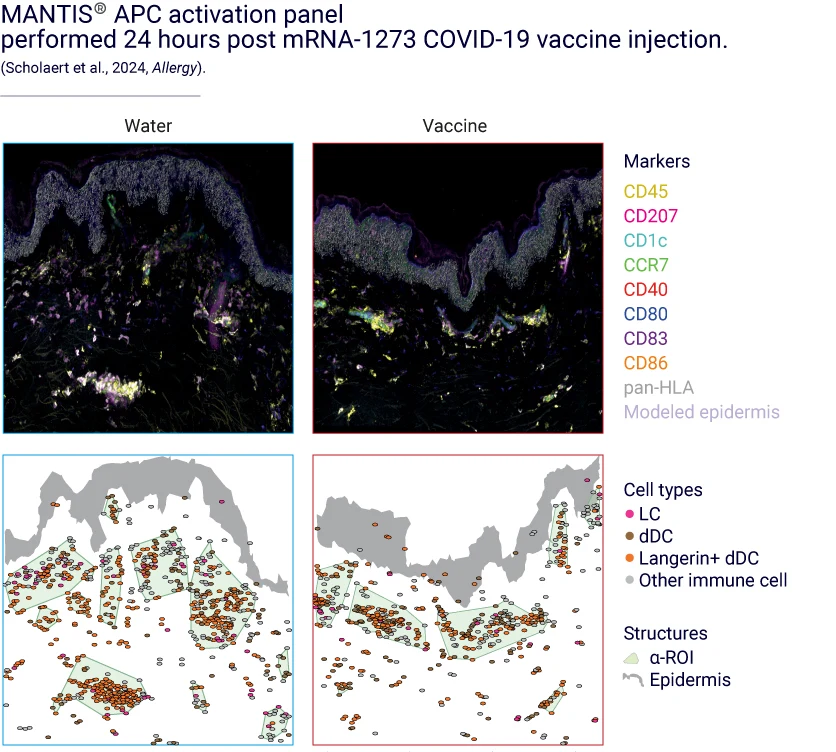
VaxSkin® leverages our proprietary spatial biology platform, MANTIS®, to study APC activation post-vaccine delivery.
This spatial biology approach provides an additional dimension to single-cell analysis by shedding light on how immune cells are arranged across all skin layers and how they interact with one another. As a result, MANTIS® enables us to track the relocation of APCs to the dermis following vaccination.
To precisely identify APCs, we have developed a nine-color panel that targets Langerhans cells (LCs), dendritic cells (DCs), and Langerin-positive dendritic cells (Langerin+ DCs). To assess the activation status of APCs, the panel incorporates cell-specific markers, including CCR7, CD80, CD83, CD86, CD40, and pan-HLA.
Frequently asked questions
How was the VaxSkin® platform validated? Can you share some relevant data using the platform?
Is VaxSkin® suitable to evaluate the best route of administration for my vaccine candidate?
Can VaxSkin® assist in the optimization of my formulation?
Scientific publications
August 2024 - Multimodal profiling of biostabilized human skin modules reveals a coordinated ecosystem response to injected mRNA-1273 COVID-19 vaccine.
Published in Allergy – August, 2024. https://doi.org/10.1111/all.16273.
Manon Scholaert, Mathias Peries, Emilie Braun, Jeremy Martin, Nadine Serhan, Alexia Loste, Audrey Bruner, Lilian Basso, Benoît Chaput, Eric Merle, Pascal Descargues, Emeline Pagès, Nicolas Gaudenzio.