Evaluation of pharmacological responses in InflammaSkin®
a fully human full-thickness ex vivo skin model reproducing key features of psoriatic lesions
USING INFLAMMASKIN EX VIVO T-CELL DRIVEN SKIN MODEL
P. Lovato (LEO Pharma), C. Jardet (Genoskin), E. Pagès (Genoskin), H. Norsgaard (LEO Pharma), P. Descargues (Genoskin)
Preclinical skin models closely mimicking inflammatory features in diseased skin, such as psoriasis, are needed to support the development of anti-inflammatory drugs in dermatology.
Approaches based on the incorporation of immune cells in reconstructed skin models are now developed. However, these skin models lack tissue complexity and diversity of immune cells and show an incomplete skin barrier function. To overcome these issues, we have developed and characterized a T cell-driven skin inflammation model (InflammaSkin®) based on the NativeSkin® explant technology and assessed pharmacological responses in this model.
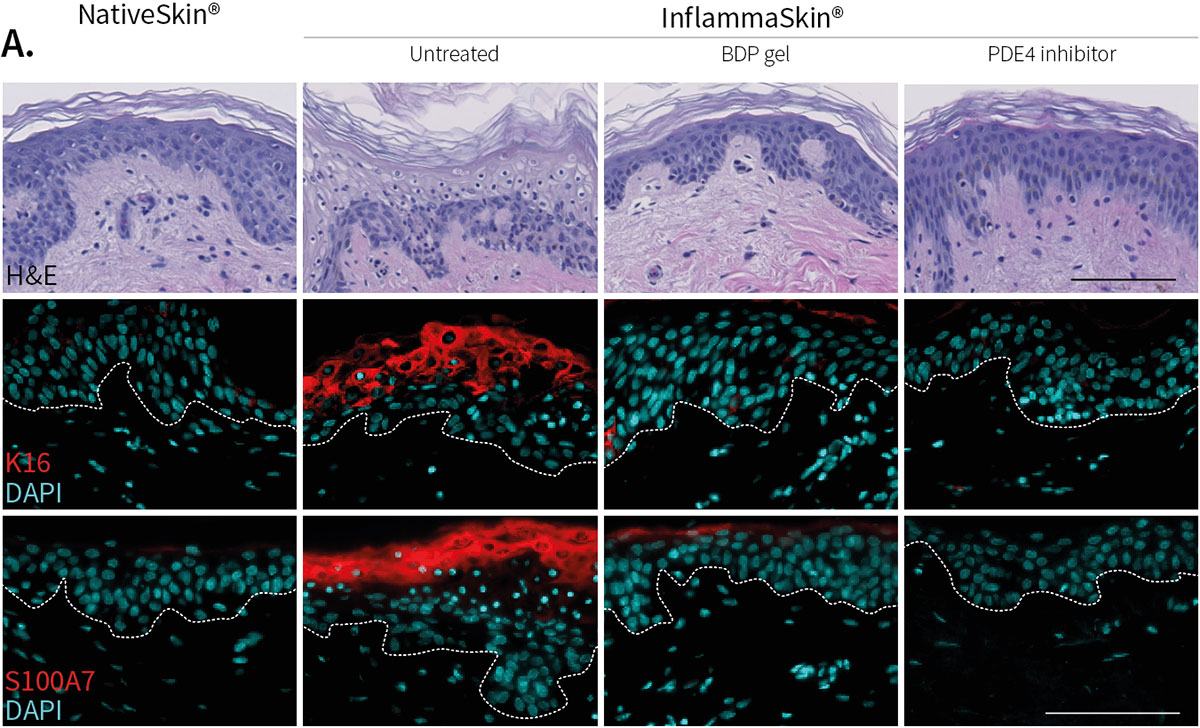
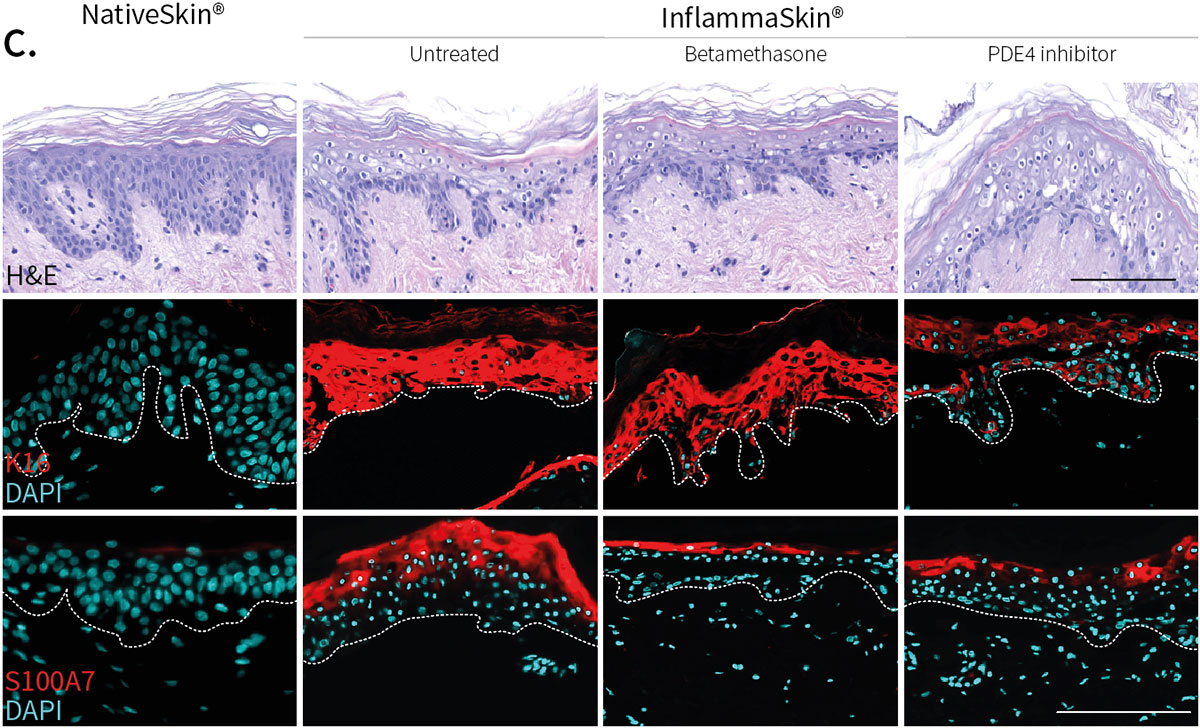
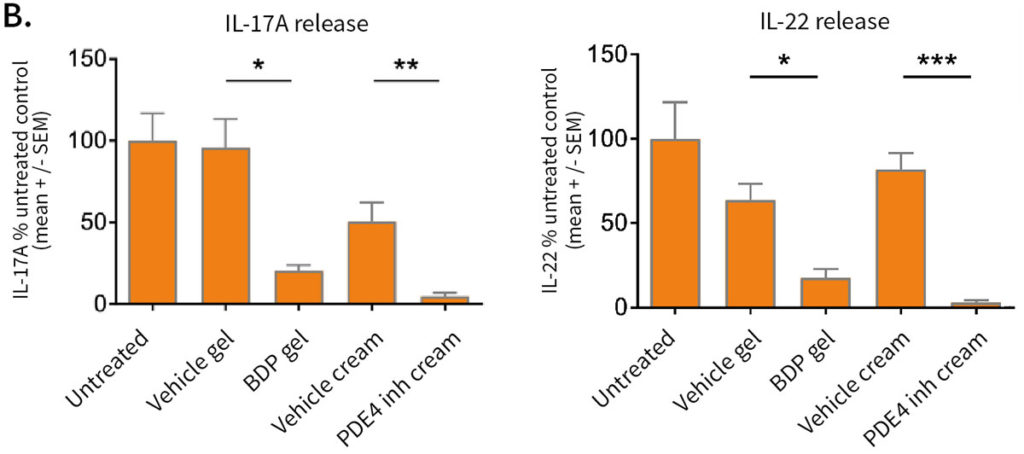
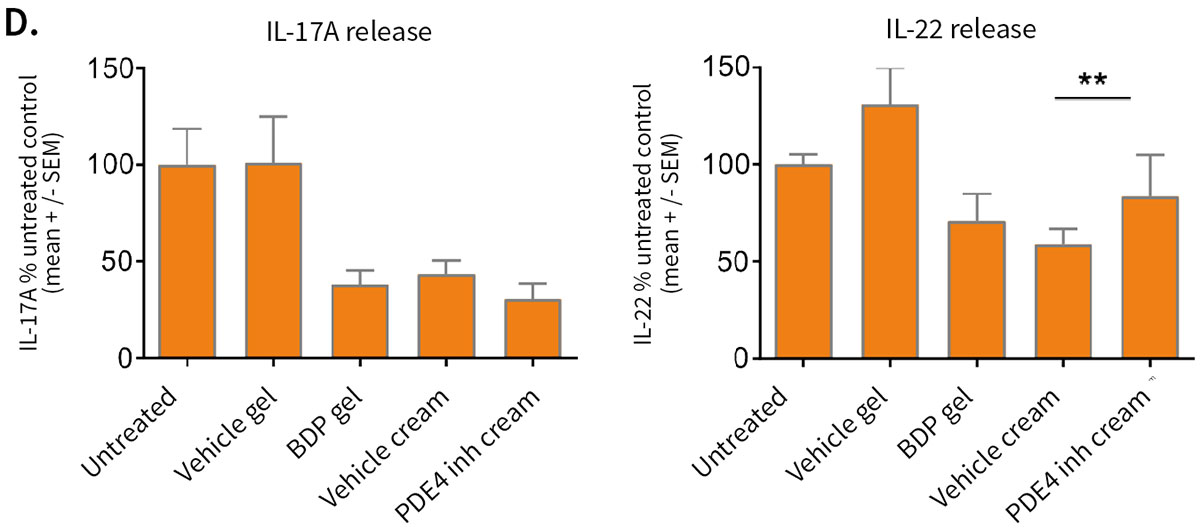
Improvement of skin structure and reduction of skin inflammation following topical treatments
Effect of topical treatment with betamethasone dipropionate or PDE4 inhibitor on the epidermal structure and activation markers and on proinflammatory cytokine release
Topical application of 10 μL Betamethasone dipropionate (BDP) gel or PDE4 inhibitor cream was performed daily for 7 days starting immediately after in situ T cell activation (prophylactic setting: panels A, B) or 4 days after activation (therapeutic setting, panels C, D). Histological analysis was performed on 5 μm-thickness formalin-fixed and paraffin-embedded skin cross-sections. Scale bars 100 μm. The level of cytokine released in the culture media was measured using specific MSD and ELISA detection kit. The results expressed as the percentage difference compared to untreated control are mean values ± SEM from independent experiments (n=3). One-way ANOVA statistical test was used to compare treatment effect by BDP and PDE4 inhibitor versus
matching vehicle control. ***P < 0.001; **P < 0.01; *P < 0.05.
Comparable results were obtained for secretion of IFN-γ.