ImmunoSafe: ISR platform®
Advancing towards prediction of clinical results
Immunotoxicity assessment with ImmunoSafe: ISR Platform®

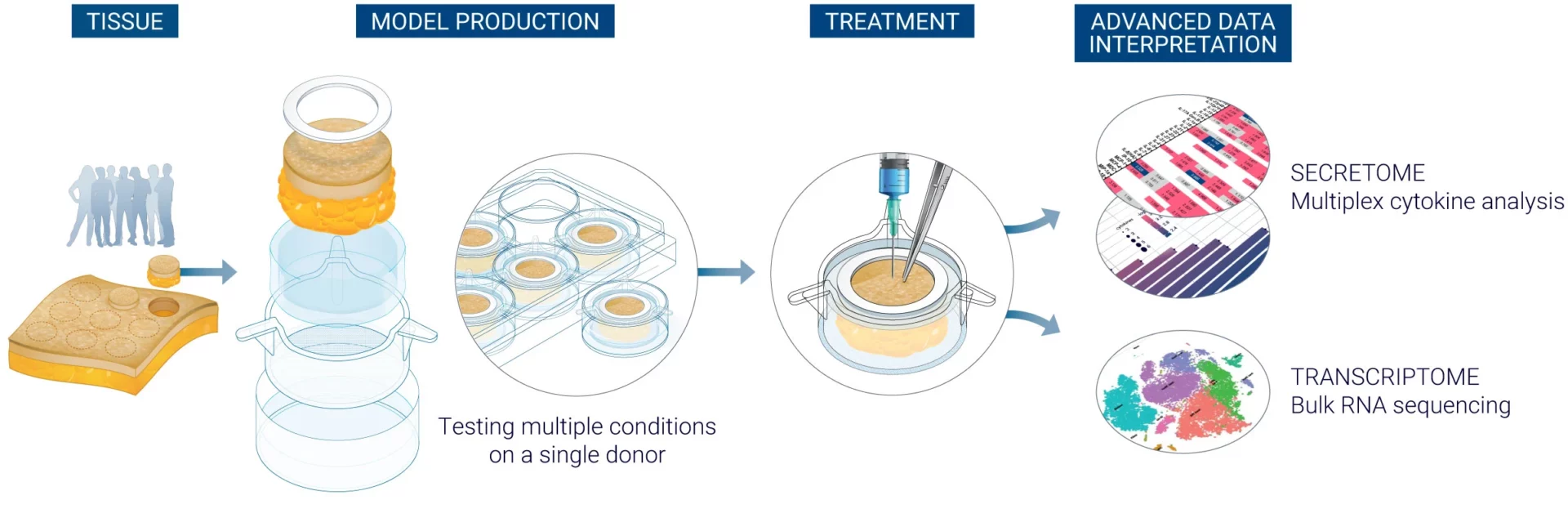
Utilizing the foundation of the ISR Platform®, the ImmunoSafe: ISR Platform offers a specialized approach to non-clinical prediction of immune-related adverse reactions. Integrating cytokine analysis from ex vivo human skin models with bioinformatics analysis, the platform supports the assessment of injection site pain and injection site reaction (ISR) of therapeutic candidates and Drug delivery systems.
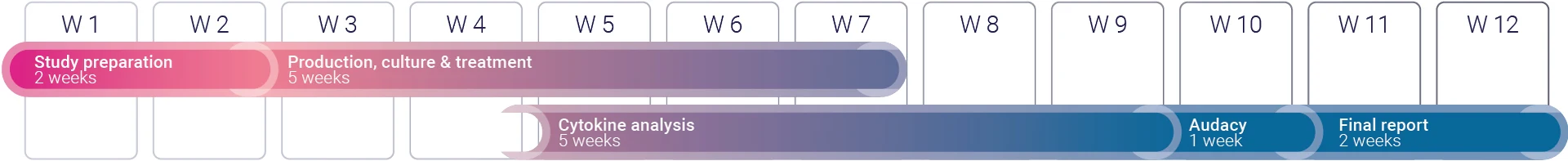
The above timeline is given for a project with 7 to 10 donors. Contact us for more information regarding the specificities of your project.
Local immunotoxicity testing to de-risk development of human therapeutics
The ImmunoSafe: ISR platform® is an innovative service engineered to reduce the risks associated with the development of injectable therapeutic agents, formulations and drug delivery systems.
Relying on cutting-edge ex vivo human skin models which are injectable and immunocompetent, the platform is designed to predict:
Injection site reactions
Injection site pain

Itch
The platform offers two powerful tools for immune response analysis: cytokine profiling and bulk RNA transcriptomic analysis. Cytokine profiling focuses on measuring secreted proteins that mediate immune responses, providing a snapshot of inflammatory response and immune activity at the site of injection. Transcriptomics analysis using bulk RNA-sequencing provides a comprehensive view of gene expression changes across all cells, offering detailed understanding of the molecular pathways activated post-injection.
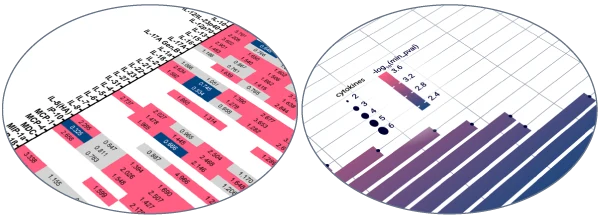
Secretomic analysis
Analysis of cytokines and chemokines released by in situ human skin resident immune cells.
Transcriptomic analysis
Analysis of bulk RNA sequencing.
After subcutaneous or intradermal treatment of models at Day 1, culture medium are sampled to perform multiplex cytokine analysis.
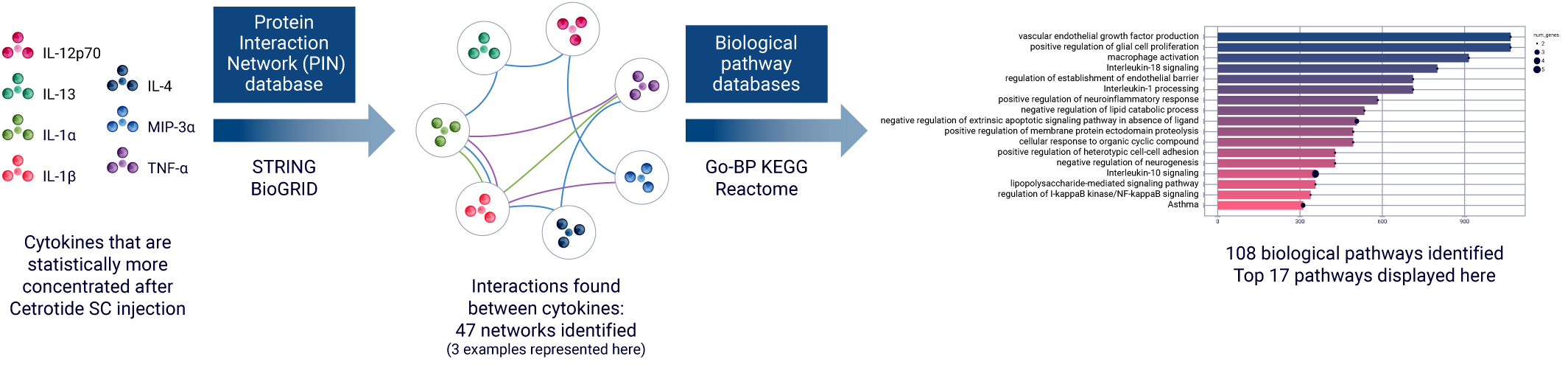
The ImmunoSafe: ISR Platform® uses models from several donors in triplicates to ensure statistically significant results of cytokine expression. This allows for more accurate characterization of the activated biological pathways implicated in the local immune response.
The exponential increase in biological data complexity has reached a point where even highly skilled experts find data analysis incredibly challenging. With this in mind, Genoskin created AUDACY (Automated Data Analysis of Cytokines), a proprietary bioinformatics-based analytical solution.
AUDACY is designed to:
- Manage large datasets and provide results quickly,
- Minimize the introduction of human error into the analysis,
- Enable novel forms of analysis.
Utilizing data from publicly available, peer reviewed online database such as PIN (Protein Interaction Network) or Reactome, AUDACY supports understanding interconnections between cytokines and gaining insight into the specific mechanisms that have been activated following the administration of the compound into the skin. Ultimately, we are able to infer on biological pathways implicated correlating the data generated ex vivo with clinically-related manifestations
Bioinformatics-based analysis of bulk RNA sequencing
The ImmunoSafe: ISR platform® is a flexible analytical workflow that also offers the possibility of bulk or single cell RNA sequencing analysis.
When discussing with our study directors, the level of detail you need regarding the detection & interpretation of adverse events after exposure to a therapeutic candidate or drug delivery system will determine the experimental design.
Below are bulk RNA sequencing data generated after subcutaneously injecting a vaccine into the HypoSkin® model.
Two timepoints at 8h and 24h showed a total or 65 and 322 differentially expressed genes (DEGs), respectively. An extended bioinformatic analysis was performed by our scientific team and using a proprietary app called FindYourPath to interpret the role of each DEG and their global implications in biological pathways
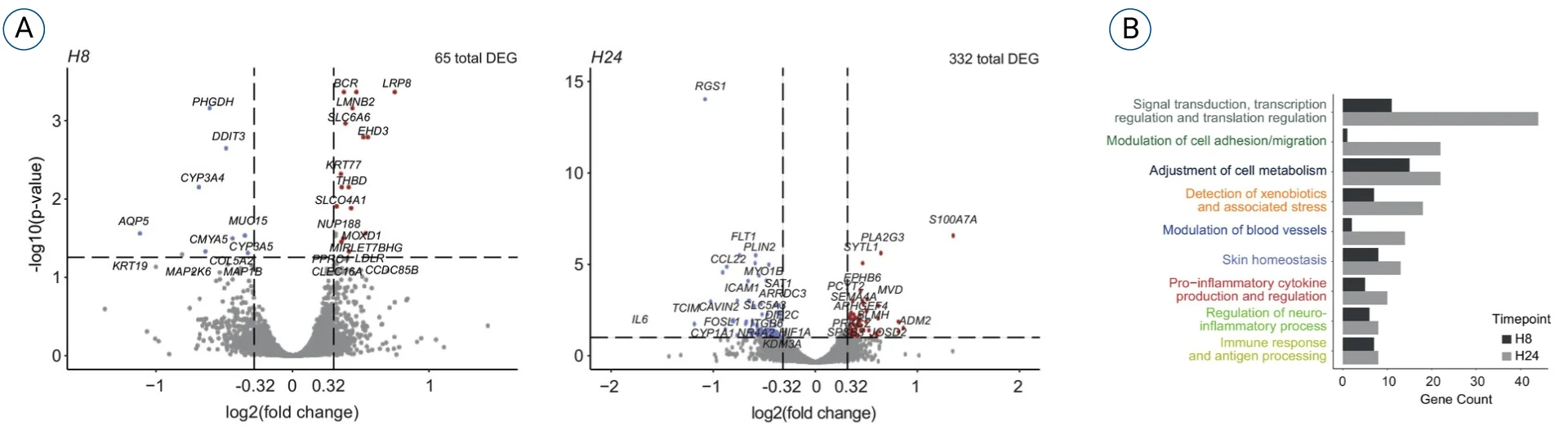
In this case, we identified 9 different categories of pathways at 8h and 24h post injection.
While cell migration and antigen processing are expected in the context of vaccine exposure, biological pathways such as the detection of xenobiotics and stress, the neuroinflammation and the blood vessel modulation could be interpreted as signs of injection site and pain reactions.
Why integrate bulk transcriptomics with cytokine analysis?
A powerful duo for immune profiling
| Bulk RNA-sequencing | Multiplex cytokine analysis |
|---|---|
| Direct quantification of gene expression | Direct quantification of proteins |
| 10,000 transcripts analyzed | ~ 30 cytokines analyzed |
| Higher sensitivity | Easier data analysis |
| Wide dynamic range of gene expression | Limited to pre-selected cytokines |
| Detection of novel target possible | Requires validated antibody |
| More complex sample preparation and data analysis | Easier sample preparation |
| Post-transcriptional regulation and protein modifications not captured |
Frequently asked questions
Who is the ImmunoSafe: ISR Platform® for?
For small molecule therapeutics that trigger local inflammation and cytotoxic tissue damage, our Local Tolerance: ISR Platform® is better suited to address these specific toxicity concerns. Both platforms provide critical data to streamline development and improve drug safety profiles.