Press corner
Everything you need to know about us and more
Genoskin uses donated human skin samples that are kept alive to generate first-in-human clinical data in order to help researchers, pharmaceutical, biotech and cosmetic companies de-risk drug & compound development.
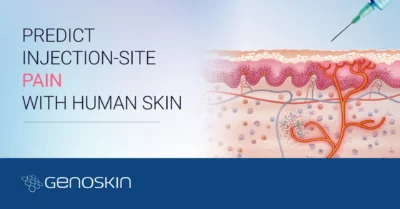
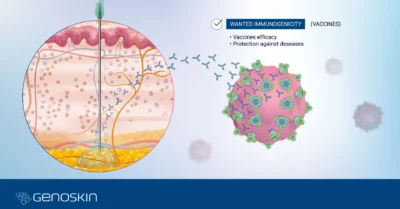
Genoskin’s live human skin models are immunocompetent and allow testing topical and systemic administrations as well as subcutaneous injections to characterize human skin reactions and immune response under multiple conditions.
The entire Genoskin team follows strict regulatory guidelines to comply with all applicable ethical and quality regulations. All Genoskin procedures and processes are ISO9001-certified.
Latest press releases

Genoskin in numbers




Executive management team








Blog