human-centric non-clinical Services
for therapeutics, vaccines, medical devices & cosmetics
Relevant human data to accelerate your drug development program
Genoskin non-clinical services leverages immunology to reduce the risks associated with drug and vaccine development, by enabling earlier access to relevant human data.

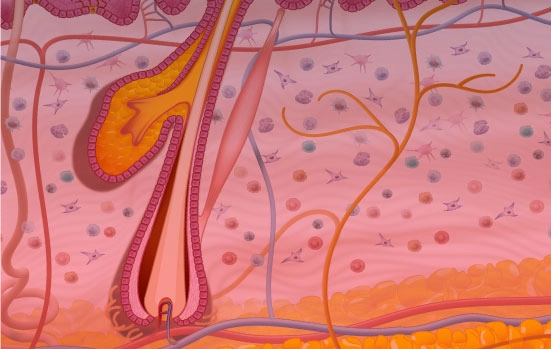
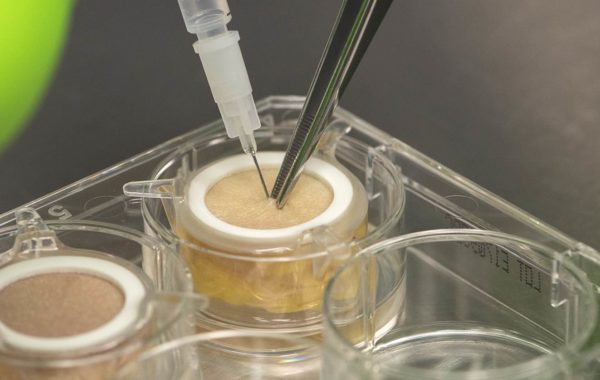
Genoskin technology uses bio-stabilized and immunocompetent ex vivo human skin from representative patient cohorts to elucidate the efficacy and toxicity of drugs and vaccines. These models are combined with advanced analytical technologies including next-generation transcriptomics, multiplexed proteomics, and bioinformatics to facilitate the generateration of actionable human data. Workflows using Genoskin technology significantly reduces the expenses, time, and risks associated with drug development, while phasing out the need for animal testing.
Drug development services
TOXICITY SERVICES
EFFICACY SERVICES
Relevant human data to accelerate your cosmetics & product development program

Genoskin uses a cutting-edge approach to support the cosmetic and consumer goods sector in validating their product claims and evaluating the efficacy and safety of cosmetic ingredients and formulations, all while ensuring no harm to humans or animals. By integrating live human skin models and expert analytical tools, we provide high-caliber non-clinical services on product efficacy that solidify your claims.
Cosmetic & consumer goods services
Different options to help you move forward
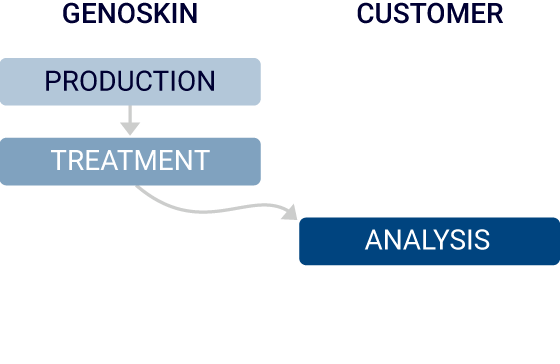
Custom pre-clinical R&D that fit your needs
Option 1
Genoskin will design the study plan according to your needs and perform a comprehensive study. The deliverable is a report with actionable insights.
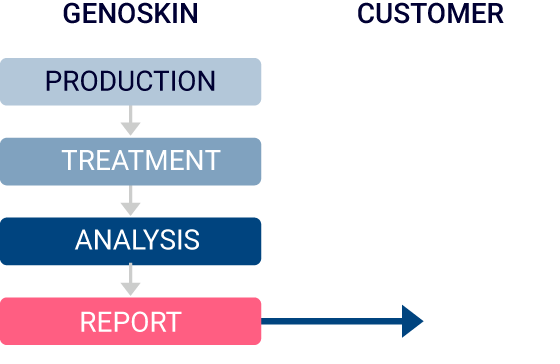
Option 2
Genoskin will tailor the study plan to meet your requirements and will administer the compound. The deliverables will be samples ready for analysis.