Ask the Expert: Your Top Questions About Genoskin’s Mast Cell Platform Answered
Curious about mast cells and their role in injection site reactions? Learn about how Genoskin sources and cultures human primary mast cells, the unique qualities of our mast cell research solution, and its diverse applications in drug toxicity assessment, allergic reaction evaluation, and vaccine development.
1. How do mast cells contribute to the development and manifestation of injection site reactions, and what mechanisms underlie their involvement in this process?
Mast cells play a pivotal role in the development and manifestation of injection site reactions through their involvement in the immune response. When a foreign substance, such as a vaccine or medication, is injected into the body, mast cells present at the injection site recognize it as a potential threat. Upon activation, mast cells release a variety of chemical mediators, including histamine, cytokines, and leukotrienes, which promote inflammation and recruit other immune cells to the area. This inflammatory response contributes to the characteristic symptoms of injection site reactions, such as redness, swelling, and pain. Additionally, mast cells can interact with other immune cells and tissue components to amplify and sustain the inflammatory process, further exacerbating the reaction.
2. What factors influence the severity and duration of injection site reactions mediated by mast cells?
The severity and duration of injection site reactions mediated by mast cells can be influenced by various factors, including the nature of the injected substance, the volume and concentration of the injected solution, the depth and location of the injection, individual variations in immune responsiveness, and any underlying inflammatory or allergic conditions. Additionally, factors such as repeated injections at the same site and the presence of adjuvants in vaccines can intensify injection site reactions.
3. How does Genoskin source and culture human primary mast cells for research purposes?
Genoskin cultivates human primary mast cells derived from peripheral blood progenitors of healthy donors. Through a specialized differentiation protocol, we generate connective tissue-type mast cells that closely resemble those found naturally in human skin. This in-house process ensures meticulous adherence to quality controls, guaranteeing the authenticity and reliability of our mast cell models for research.
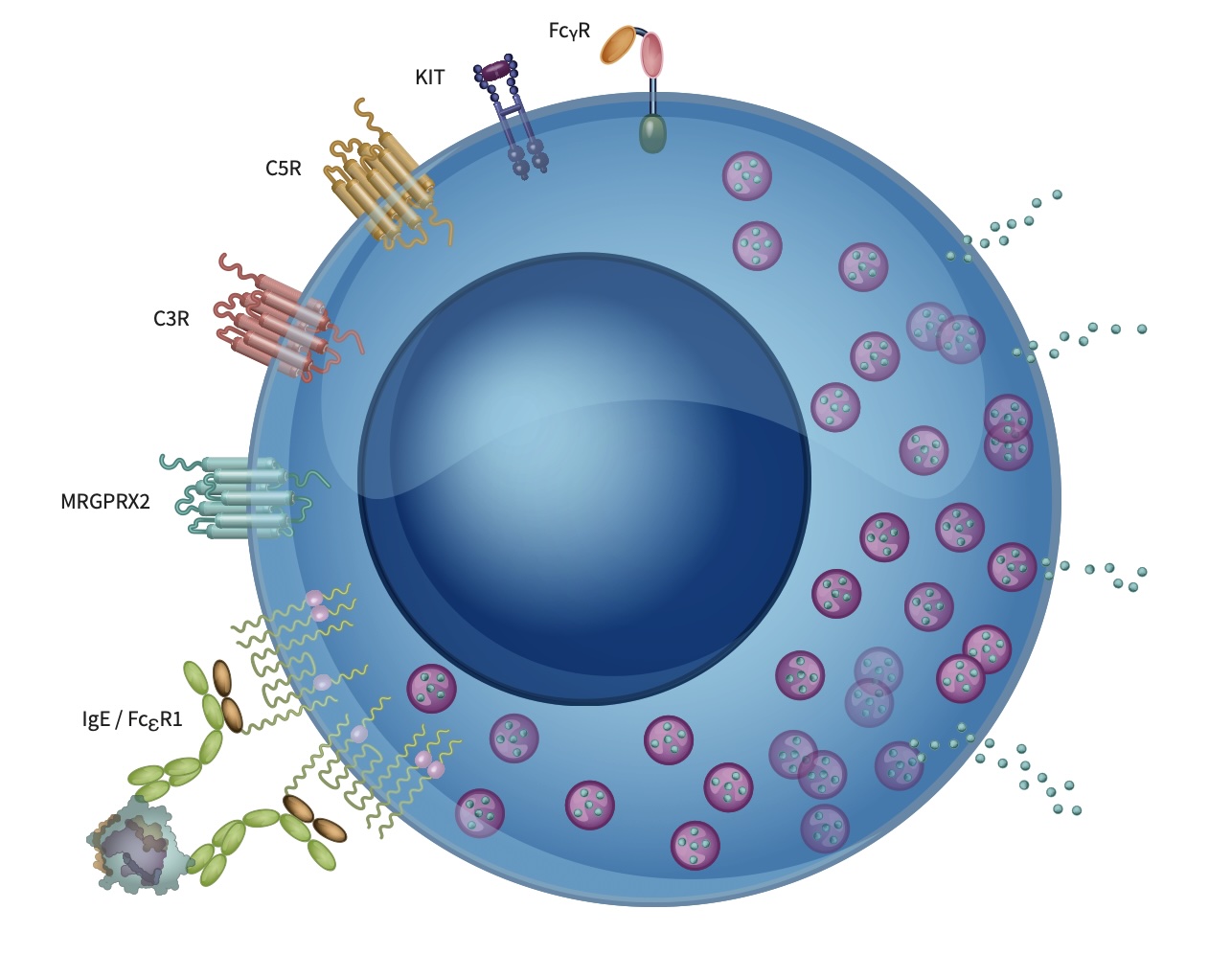
Figure: Mast cells play critical roles in immune responses and disease pathogenesis. Mature mast cells contain granules storing various mediators and are activated through multiple receptors. Here, we illustrate a mast cell with receptors including FcϵRI, MRGPRX2, c-kit, C3R, and C5R, highlighting their importance in mediating mast cell responses to environmental stimuli and pathogens.
4. What makes Genoskin’s solution unique for mast cell research?
Genoskin’s solution stands out for mast cell research due to its utilization of connective-tissue type human primary mast cells in an exclusive in-house assay, offering a unique approach to studying in vitro drug-induced mast cell degranulation and inhibition. By sourcing mast cells from peripheral blood progenitors of healthy donors and employing a specialized differentiation protocol, Genoskin generates connective tissue-type mast cells that resemble those naturally found in human skin. This ensures authenticity and relevance in drug development studies. With meticulous adherence to functional and quality controls at every stage, Genoskin’s human primary mast cell services enable comprehensive assessments of drug efficacy and toxicity, providing invaluable insights into potential allergic reactions and desired immune responses, ultimately advancing pharmaceutical research and development.
5. What specific applications can the Genoskin mast cell platform be used for?
Our mast cell platform is versatile and can be utilized for various purposes, including drug toxicity assessment, evaluation of allergic reactions induced by drug candidates, and investigation of drug efficacy. By monitoring mast cell degranulation, researchers can assess potential drug toxicity, allergic response induction, and the effectiveness of drug candidates in triggering immune responses, particularly in areas such as immunotherapy and vaccine development.This includes assays such as β-hexosaminidase assays, histamine assays, and cytokine release analysis.
6. Can mast cells contribute to the modulation of immune responses induced by vaccines administered via injection?
Yes, mast cells can influence the immune responses elicited by vaccines administered via injection. Through their ability to release cytokines and other signaling molecules, mast cells can modulate the activity of other immune cells, such as dendritic cells, T cells, and B cells, at the injection site. This can impact the magnitude and nature of the immune response generated against vaccine antigens, potentially influencing vaccine efficacy and the development of immunological memory.
7. Can Genoskin tailor custom studies using the mast cell platform to meet specific research needs?
Absolutely. At Genoskin, we are committed to advancing research goals by offering tailored solutions. We can customize studies using our mast cell platform to address specific research questions or objectives, ensuring that researchers have the tools and resources they need to achieve their scientific goals effectively and efficiently.
Comments are closed.