WoundSkin® ex vivo skin model
to study wound healing & bacterial colonization
WoundSkin®
ex vivo skin model
to study wound healing & bacterial colonization
WoundSkin®
ex vivo skin model
to study wound healing & bacterial colonization
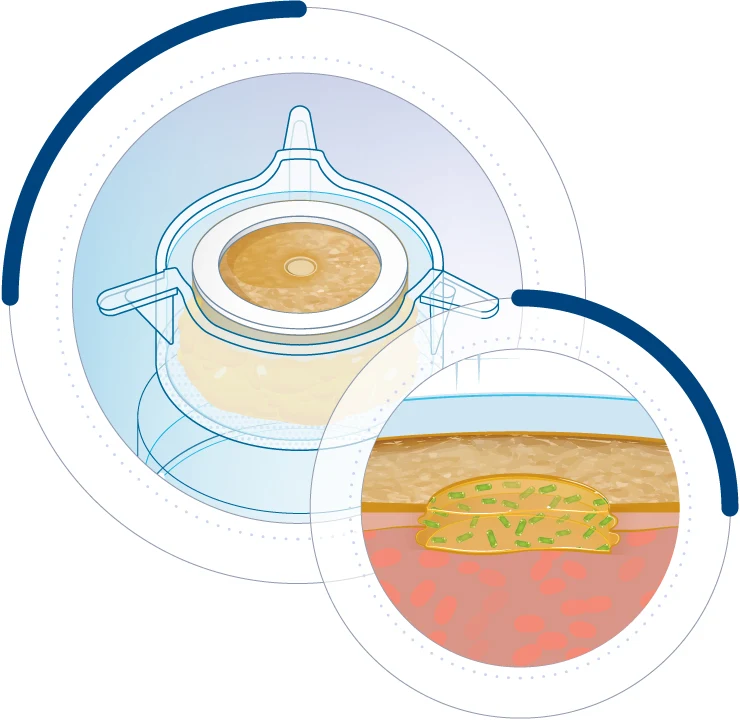
WoundSkin®: unique wounded human skin model
Immunocompetent ex vivo model to study the efficacy of compounds against wound infection

WoundSkin® is a physiologically relevant ex vivo human skin model designed to study wound healing and wound infection in a controlled environment. This innovative platform allows for the evaluation of topical therapies and dressings, whether in a prophylactic setting to prevent infection or as a therapeutic treatment for already infected wounds. WoundSkin® provides a reliable tool to assess the efficacy of wound care formulations and dressings, supporting the development of advanced solutions for faster healing and improved infection control. A quick overview of the model’s features and benefits:
Immunocompetent
WoundSkin® models hold the features of in vivo human skin, including a functional immune system that responds to bacterial infection, for predictive human data.

Wounded
The models contain 2 mm wide – circular wound allowing the study of wound healing and bacterial infection processes.
Skin
WoundSkin® models are standardized to allow for reproducible research and testing on real, live human epidermis and dermis.
On-demand
WoundSkin® is available on-demand, with models shipped globally to support your in-house studies without delay.
Why study wound healing & bacterial colonization?

Skin possesses excellent regeneration properties that allows its rapid healing upon dermal injury. If wounds fail to heal in an orderly and timely manner, chronic ulcers (e.g. diabetic foot ulcers, venous leg ulcers, pressure ulcers, etc.) develop. Efficient treatment of chronic wounds remains a global challenge. Next to wound debriding or hyperbaric oxygen therapy, a myriad of topical therapies and dressings are available to the clinicians with very few having shown their effectiveness in promoting wound repair. The integration of nanomaterials into wound healing bandages is believed to be one possibility to overcome some of the current limitations. To test the potential of these innovative wound healing dressings, wound infection models are needed. Current research on wound infections is mainly conducted on animal models, which often limits direct transferability to human and poses ethic issues.
WoundSkin®: wound healing & microbial infection
Unique technology for reliable data

WoundSkin® is built on Genoskin’s patented NativeSkin® technology, using live, ex vivo human skin sourced from donated surgical discard. Each model contains a precisely controlled wound, created by mechanically removing the epidermis and part of the dermis, making it ideal for studying wound healing and infection.
WoundSkin® is fully immunocompetent, enabling the assessment of compounds for both infection prevention and treatment. Designed with a silicone ring, the model prevents lateral bacteria or formulation leakage, ensuring reliable and controlled infection studies.
Scientific validation: WoundSkin®, a model to study wound infection and wound healing
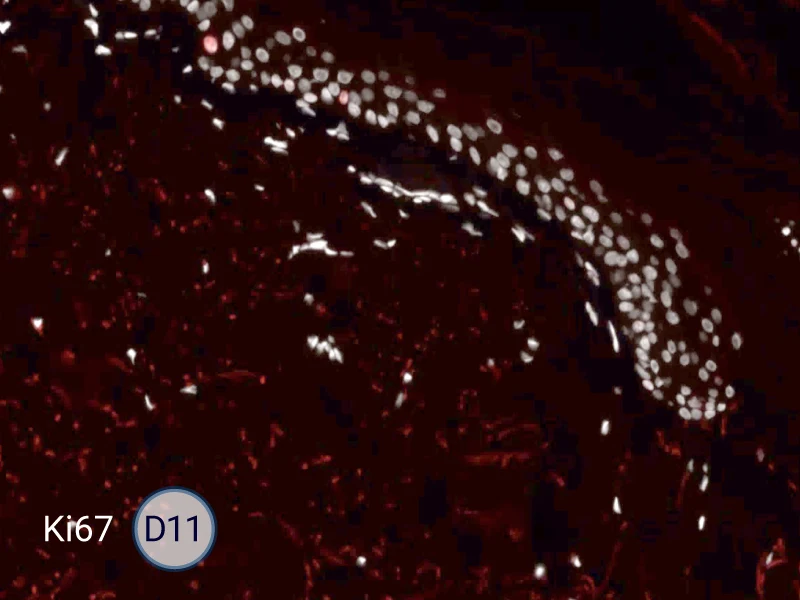
Characterization of epithelial tongue
Upon injury, growth factors like EGF and TGF-β activate keratinocytes, reducing adhesion and triggering migration. These cells extend lamellipodia and move as a cohesive sheet over the wound bed, forming the epithelial tongue. The formation of the epithelial tongue is a key step in re-epithelialization. Studying epithelial tongue formation in ex vivo skin models like WoundSkin® enables the evaluation of topical therapies and dressings that can enhance re-epithelialization, making it a key feature in wound healing research.
As WoundSkin® is real human skin, it contains all the necessary features to heal and form an epithelial tongue.
The evolution of the wound without treatment has been followed over 11 days by histology and immunofluorescence. The data are showing the closing of the wound over time.
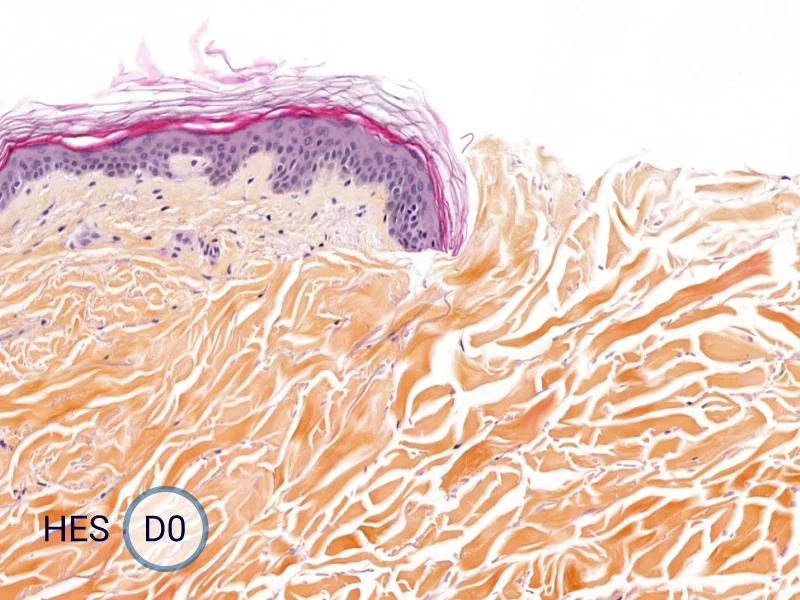
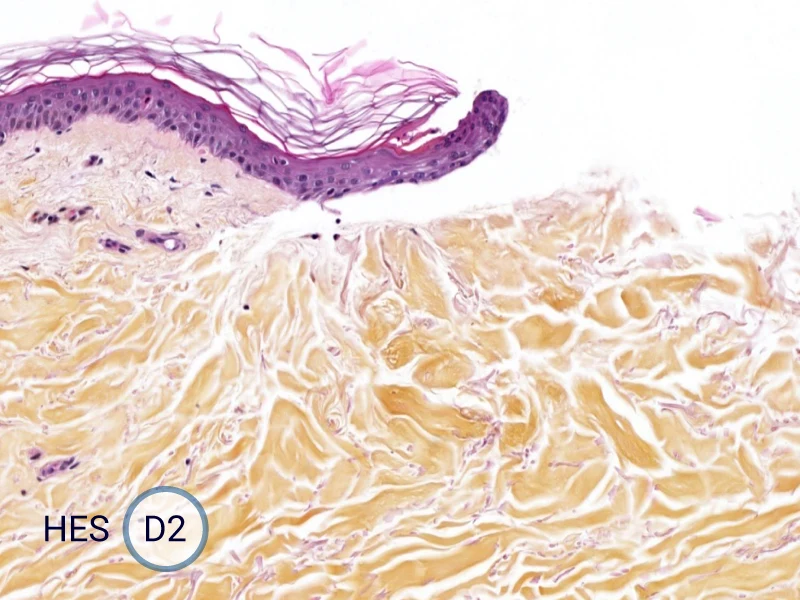
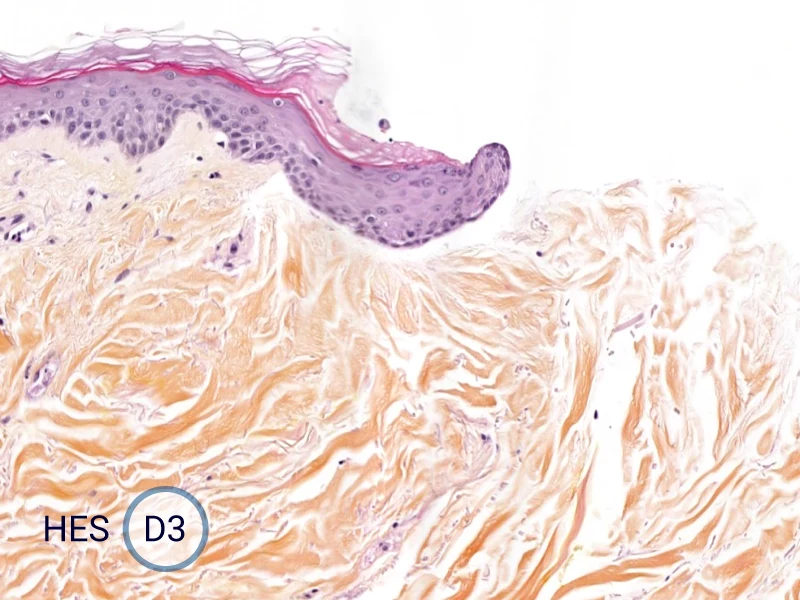
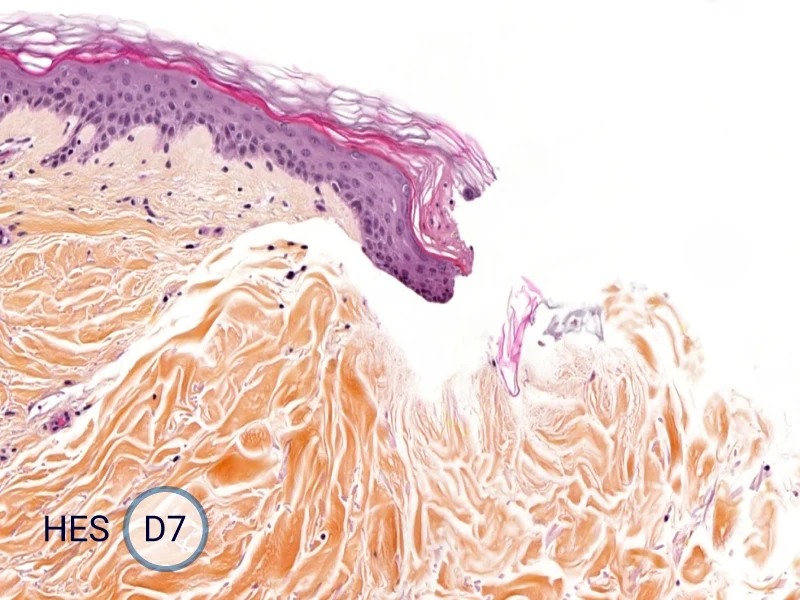
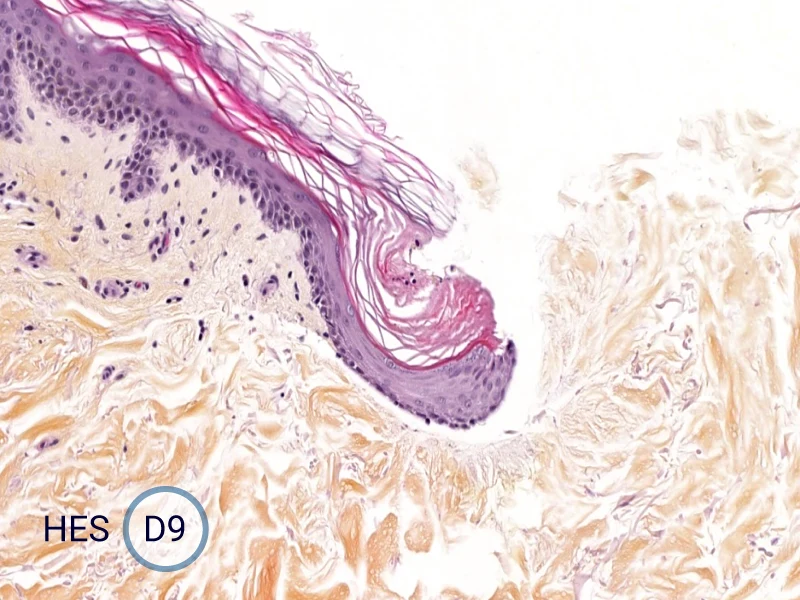
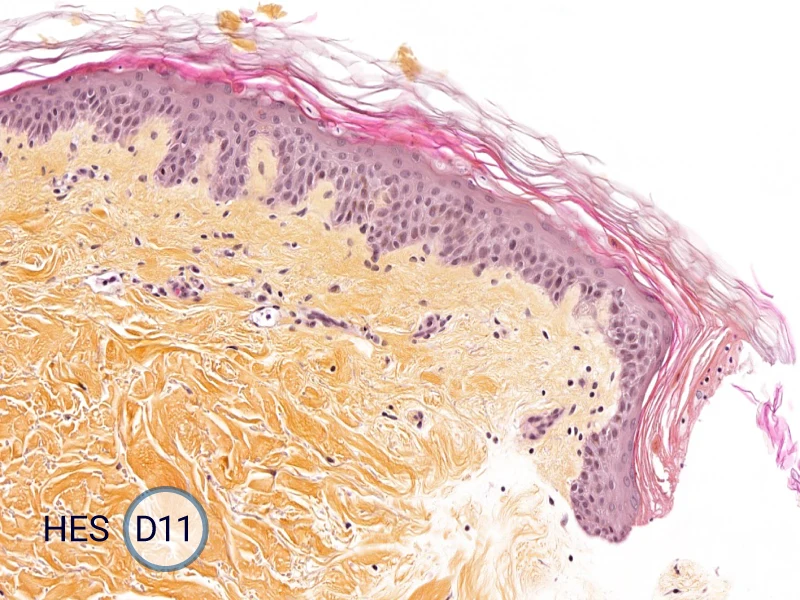
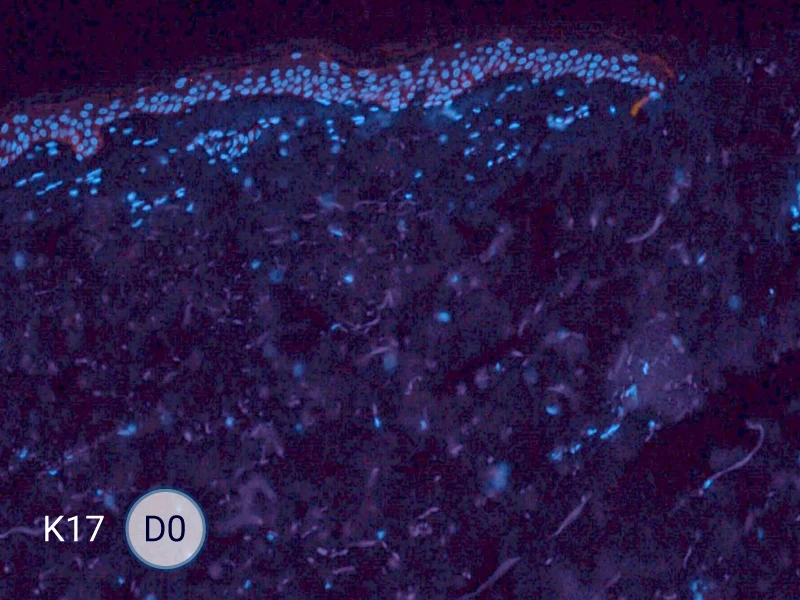
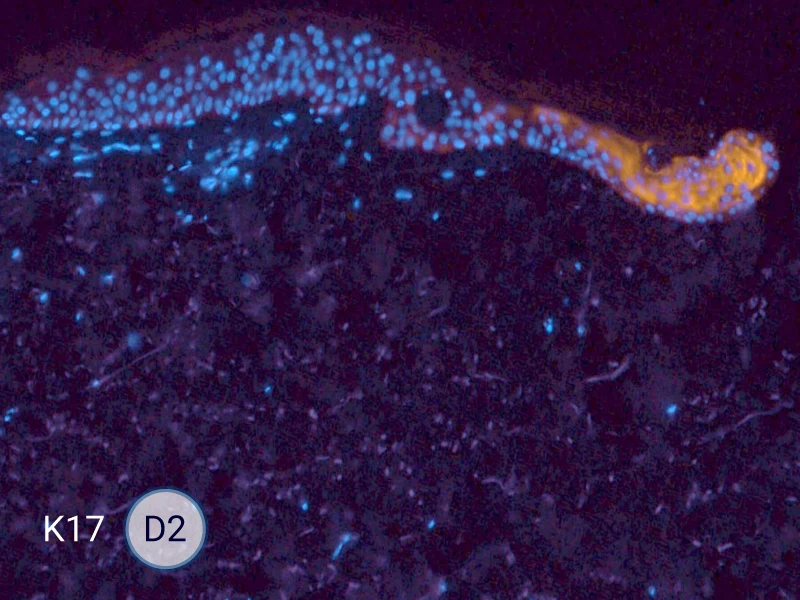
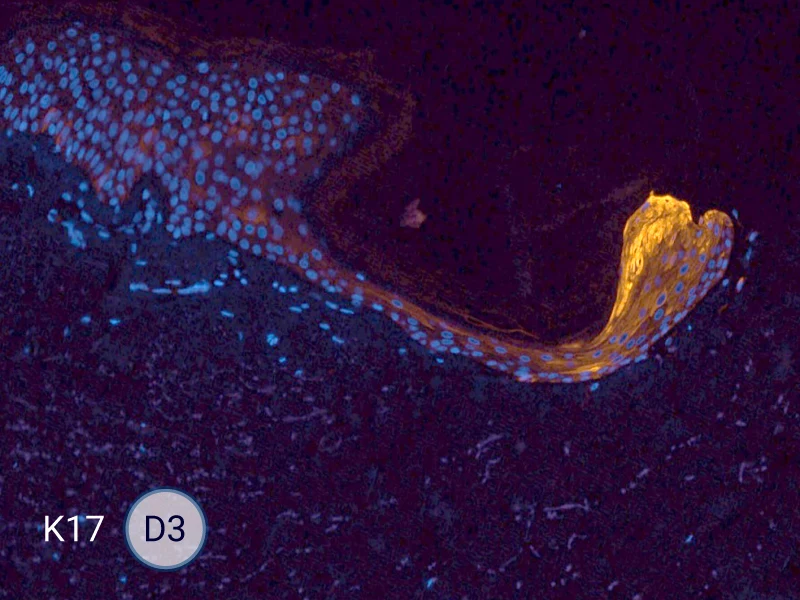
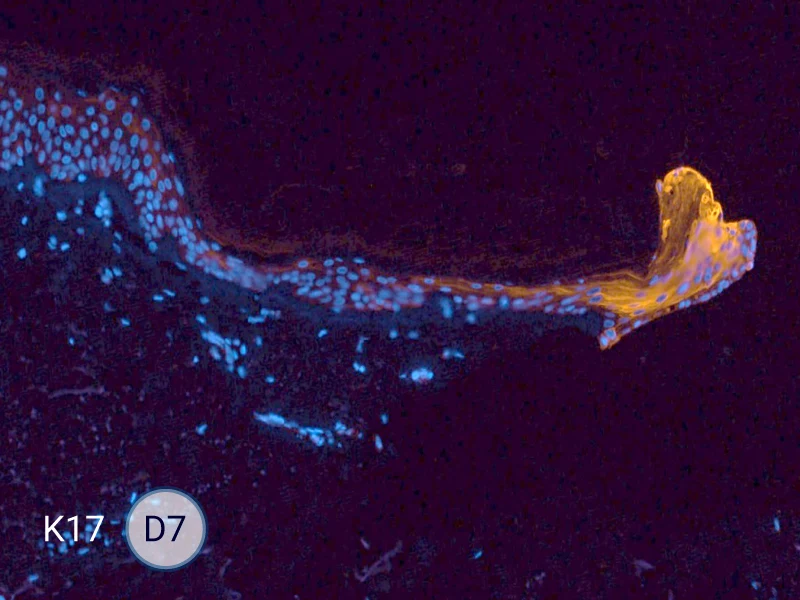
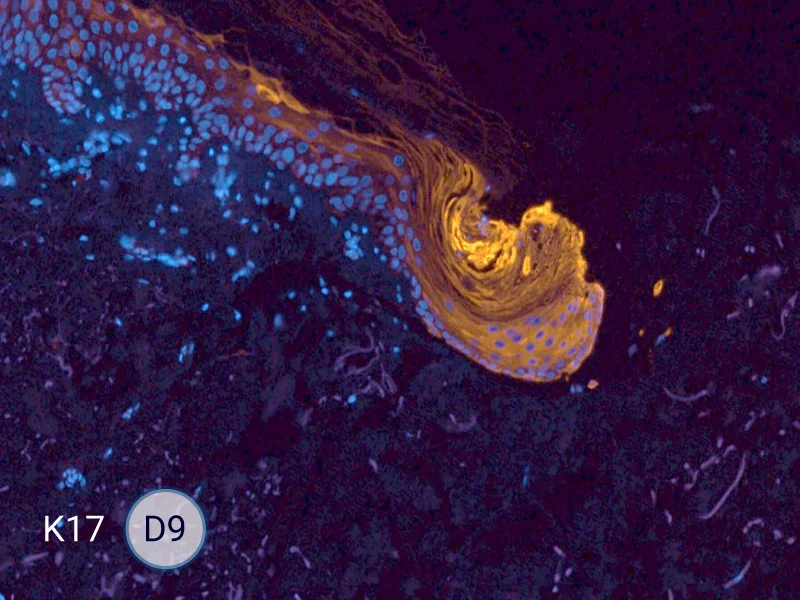

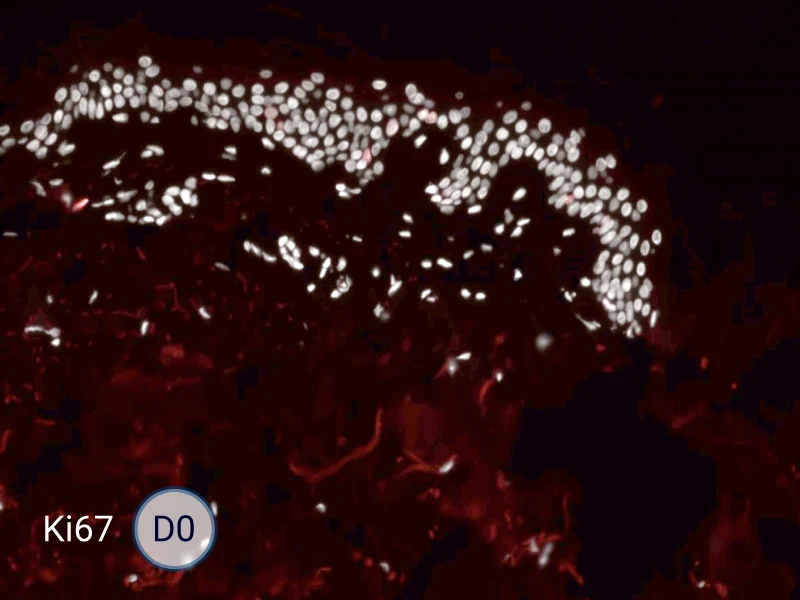
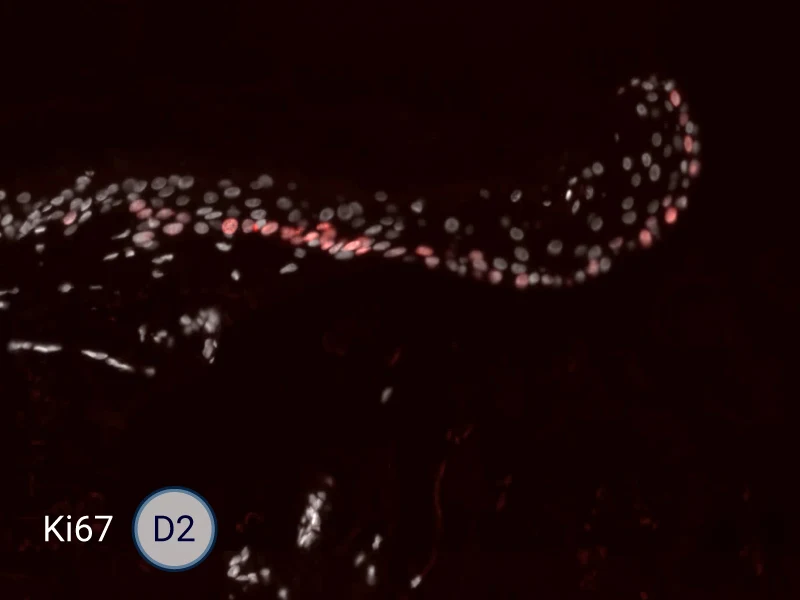
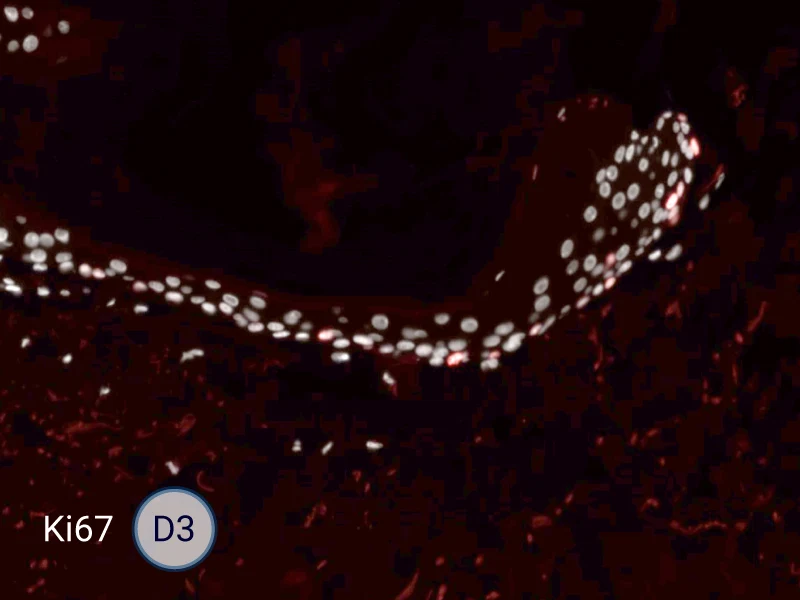

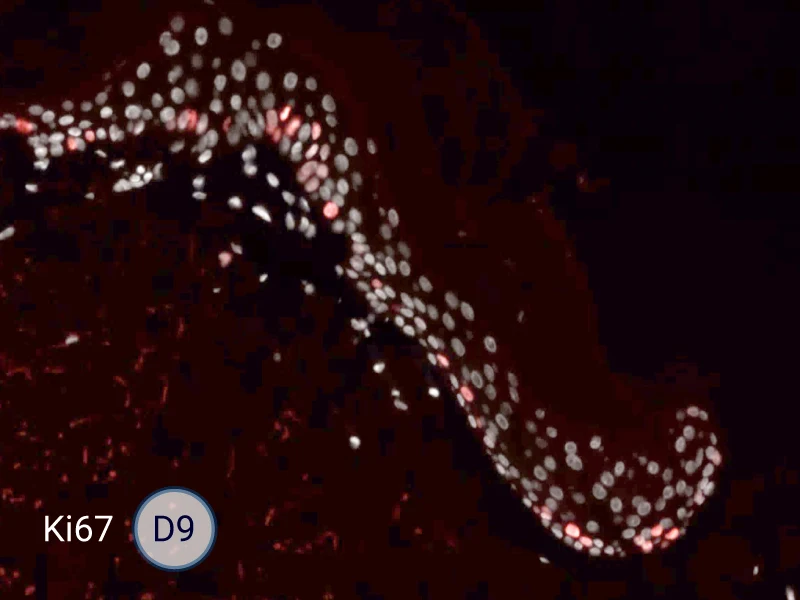
HES (Hematoxylin-Eosin-Safran) shows the progression of the epithelial tongue.
K17: specific marker of keratinocytes migrating over to wound bed.
Ki67: cells in the epithelial tongue are proliferating.
Validation of wound infection by S. aureus bacteria
In order to study wound infection, bacterial infection was initiated by dropping S. aureus (105 to 2×107 CFU) solutions on the wound of WoundSkin® models and keeping the skin samples in culture medium without antibiotics at 37°C for 4 days. The wound was then analyzed for the presence of infection.
On average, the detected amount of bacteria in infected skins was 1×108 CFU/g of skin. CFU = colony forming units
Skin containing S. aureus bacteria
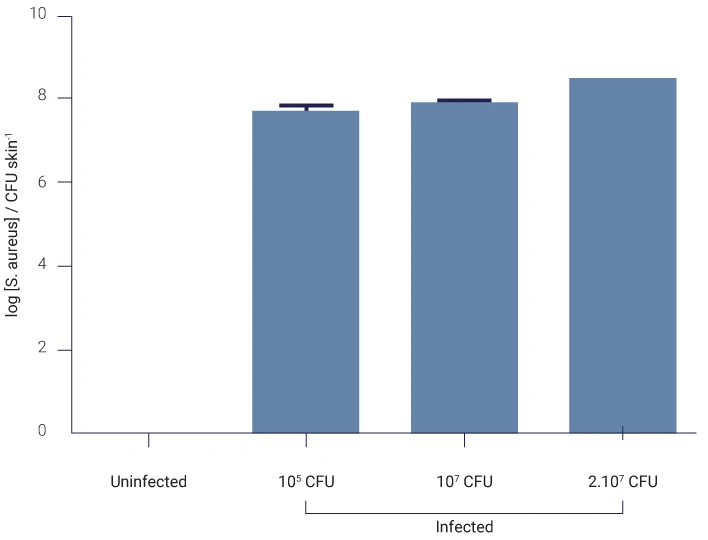
Number of bacteria colony forming units (CFU) in skin infected for 4 days by different concentrations of S. aureus is shown on the graph. No bacteria were detected on uninfected skin (control).
CFU/g of skin.
Analysis of the biofilm development
To study the development of a wound infection, a bacterial infection was initiated by dropping S. aureus solutions on the wound.
After bacterial infection of WoundSkin® models, the biofilm development has been studied by hematoxylin and eosin staining and immunofluorescence. The induced infection has all the features of a wound infection including biofilm development.
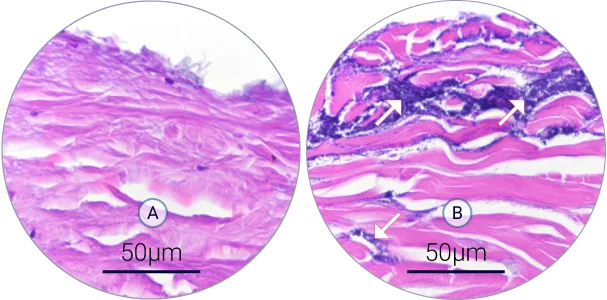
Biofilm development on the wound (A-B)
Hematoxylin and eosin staining shows the structure of the wound (dermis in pink) and bacteria (dark purple). A S. aureus biofilm developed over 4 days of infection in the wound.
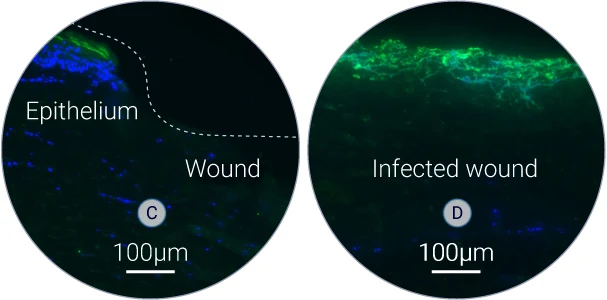
S. aureus specific biofilm (C-D)
S. aureus was detected on tissue sections by using a specific antibody. High level of green fluorescence indicates the presence of S. aureus within the wound.
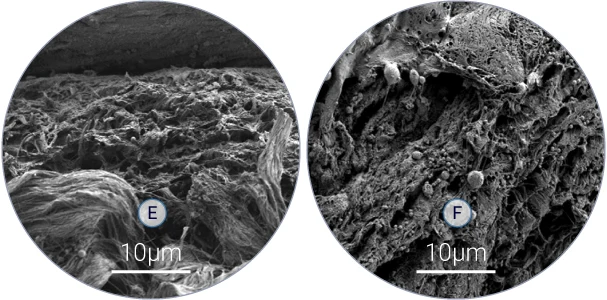
Bacterial colonization of skin tissue (E-F)
Scanning electron microscopy analysis shows presence of bacteria among collagen fibers.
Skin Inflammation in Response to Staphylococcus aureus Colonization
The inflammatory response has been studied by analyzing the expression of different pro-inflammatory cytokines.
The analysis shows an increase of cytokines expression levels after 4 days post-infection with S.aureus, confirming the initiation of inflammatory responses after bacterial infection.
S. aureus induces inflammatory response
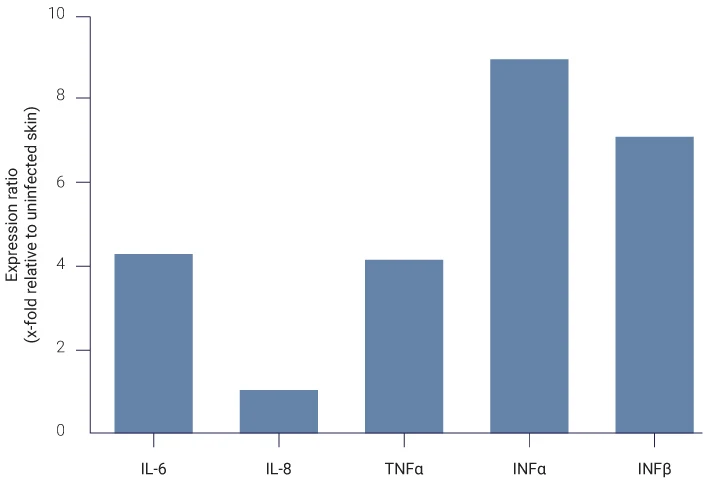
Analysis of pro-inflammatory cytokines (IL-6, IL-8, TNFα, INFα, INFβ) expression by qPCR on total skin lysates.
Resources

February 2025 - Effects of Chlorella protothecoides-derived polydeoxyribonucleotides on skin regeneration and wound healing
Published in Dermatological Research, February 2025;Arch Dermatol Res 317, 483 (2025). https://doi.org/10.1007/s00403-025-03885-w
Park, J.M., Nam, G.B., Lee, ES. et al.
June 2023 - Wound healing phases
Published in StatPearls, June 2023.
Heather A. Wallace; Brandon M. Basehore; Patrick M. Zito.
July 2022 - Nanobiotechnology: applications in chronic wound healing
Published in Dovexpress, July 2022, Volume 2022:17 Pages 3125—3145. https://doi.org/10.2147/IJN.S372211
Tao Jiang, Qianyun Li, Jinmei Qiu, Jing Chen, Shuang Du, Xiang Xu, Zihan Wu, Xiaofan Yang, Zhenbing Chen, Tongkai Chen
February 2022 - Nanomaterial-based therapy for wound healing
Published in MDPI, February 2022;12(4):618. doi: 10.3390/nano12040618.
Anamika Kushwaha, Lalit Goswami, Beom Soo Kim
January 2021 - Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis
Published in Journal of Nanobiotechnology, January 2021, volume 19, Article number: 1. https://doi.org/10.1186/s12951-020-00755-7
Hamed Nosrati, Reza Aramideh Khouy, Ali Nosrati, Mohammad Khodaei, Mehdi Banitalebi-Dehkordi, Korosh Ashrafi-Dehkordi, Samira Sanami & Zohreh Alizadeh.
Novembre 2018 - Wound healing: a cellular perspective
Published in Physiological Review, 2018, 99: 665–706. https://doi.org/10.1152/physrev.00067.2017
Melanie Rodrigues, Nina Kosaric ,Clark A. Bonham and Geoffrey C. Gurtner
September 2018 - Poster - Bacterial infection and wound healing in an ex vivo human skin model
Scientific Poster presented at the 2019 Congress of the European Society for Dermatological Research (ESDR) – September 18-21, 2019
Pagès E, Pastore M, Rosselle L, Barras A, Skandrani N, Cantelmo, A.R, Boukherroub R, Descargues P, Szunerits, S
June 2013 - The molecular features of tongue epithelium treated with the carcinogen 4-nitroquinoline-1-oxide and alcohol as a model for HNSCC
Published in Carcinogenesis, June 2013 ;34(11):2673–2681. doi: 10.1093/carcin/bgt223
Kwame Osei-Sarfo, Xiao-Han Tang, Alison M Urvalek, Theresa Scognamiglio, Lorraine J Gudas.
March 2010 - Factors affecting wound healing
Published in Journal of Dental Research, March 2010, 89(3):219-229. doi: 10.1177/0022034509359125.
Guo S, Dipietro LA.