Top things found in human skin (vs in reconstructed human skin models)
Covering nearly two square meters, skin is our body’s largest organ and a common pathway for administering drugs—whether through topical applications, subcutaneous injections, intradermal routes, or medical devices. With its complex network of immune cells, the skin plays an essential role in testing the efficacy and safety of pharmaceutical and cosmetic products. Today, these studies are commonly conducted on animal skin, reconstructed skin, or real human skin maintained ex vivo. In a previous blog, we explored the main differences between animal and human skin. Now, let’s dive into the key differences between reconstructed and real human skin.
How are reconstructed human skin models used in research today?
Reconstructed human skin models emerged in the 1980s as an ethical and more accurate alternative to animal testing1. Designed to provide a reliable platform for research, these models are now a mainstay in cosmetic research, helping to assess the safety and efficacy of topical products. As some countries have banned animal testing in cosmetics and consumer goods, the demand for reconstructed skin models has increased. Globally, regulatory agencies are moving away from animal testing, supported by initiatives like the FDA Modernization Act. In the EU, reconstructed skin models are utilized in tests validated by the OECD, such as those for skin corrosion, irritation, and dermal absorption. They are also valuable for studying potential allergic reactions.
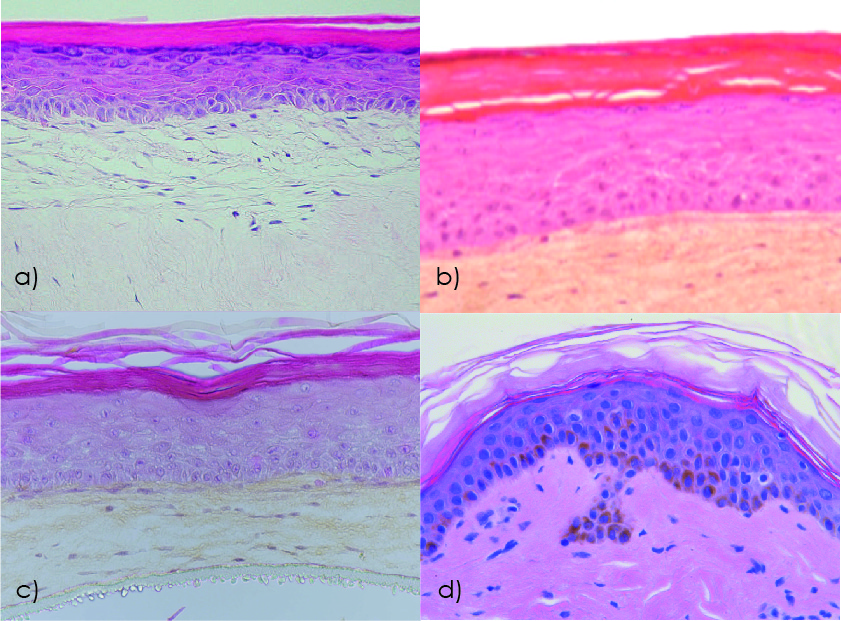 Figure 1. Human skin model histology studied by hematoxylin-eosin staining: a) Reconstructed human skin model EpiDermFT (MatTek); b) Reconstructed human skin model T-Skin (Episkin); c) Bioprinted human skin model Poieskin® (Poietis); d) Ex vivo human skin explant NativeSkin® (Genoskin). Modified from 2 and 3.
Figure 1. Human skin model histology studied by hematoxylin-eosin staining: a) Reconstructed human skin model EpiDermFT (MatTek); b) Reconstructed human skin model T-Skin (Episkin); c) Bioprinted human skin model Poieskin® (Poietis); d) Ex vivo human skin explant NativeSkin® (Genoskin). Modified from 2 and 3.
Over time, these models have evolved in complexity (Figure 1), and now, advancements like 3D bioprinting and skin-on-a-chip technologies are paving the way for even more sophisticated models. But how accurately do these models represent real human skin? And can they fulfill the needs of dermatology and drug development?
What are the top 10 differences between reconstructed and real human skin?
Skin basic structure
Human skin is a complex organ composed of three main layers:
- The epidermis is the outermost layer, primarily made up of keratinocytes arranged in five sublayers. This layer provides the first protective barrier against external elements.
- The dermis, located beneath the epidermis, consists of connective tissue containing blood vessels, hair follicles, sweat glands, sebaceous glands, nerve endings, collagen and elastin fibers, and immune cells. This layer provides structural support and elasticity.
- The hypodermis is the deepest layer, composed of loose connective tissue, adipocytes, and larger blood vessels and nerves.
Standard full-thickness reconstructed human skin models consist of a dermal equivalent overlaid by a stratified epidermal equivalent. To create the dermis, primary fibroblasts are seeded in a collagen matrix to form a supportive layer that allows for cell-cell interactions. Primary human keratinocytes are then cultured on top of this living dermal equivalent, where they differentiate into 8-12 layers and form a stratum corneum at the tissue/air interface.
The main structural difference between these models and native human skin lies in the absence (Figure 2) of a natural dermis with papillary and reticular layers, as well as the absence of rete ridges at the dermal-epidermal junction.
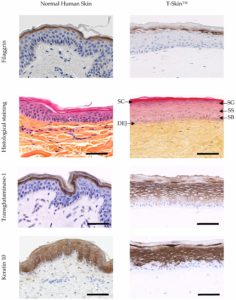
Figure 2. Characterization of the epidermis from normal human skin and reconstructed human skin (T-Skin™) by histology and immunohistochemistry (anti- keratin 10, Transglutaminase-1, Filaggrin) analysis on paraffin sections. Localization on histological stained images of the stratum corneum (SC), stratum granulosum (SG), stratum spinosum (SS), stratum basal (SB) layers of the epidermis and the dermo-epidermal junction (DEJ). Scale bar represents 100 μm. Modified from 3.
Presence of subcutaneous tissue
The hypodermis, or subcutaneous layer, lies below the dermis and functions as an endocrine organ crucial for glucose homeostasis and lipid metabolism.
Primarily composed of fibrocytes and adipocytes, the hypodermis is rich in proteoglycans and glycosaminoglycans. Known for storing energy in fatty acids and serving as an insulating layer, the hypodermis also plays an essential role in immune defense by producing mediators like growth factors, adipokines, cytokines, and chemokines, which are released into circulation.
Subcutaneous injections leverage this layer for effective drug delivery, as the hypodermis can store injected compounds and release them gradually into the bloodstream.
Currently, no commercially available reconstructed skin models include subcutaneous tissue. Researchers are exploring different approaches to reconstructing 3D adipose tissue, using adipose-derived stem cells (ASCs) differentiated into adipocytes, or isolating mature adipocytes from liposuction samples to construct a functional subcutis.
Robustness to support injection
Several routes are available for drug administration through the skin. The most common injection types are subcutaneous (targeting the hypodermis) and intradermal (targeting the dermis). Transdermal delivery, which uses patches to deliver drugs through the skin into systemic circulation, is also widely used. Recently, innovative skin delivery methods have emerged, including microneedles that create microchannels for drug delivery without reaching pain receptors. Additionally, a variety of medical devices are applied to or injected into the skin.
Since full-thickness reconstructed skin models lack a hypodermis layer, they are not suitable for subcutaneous injection. To date, no studies have been conducted on intradermal injections in reconstructed skin, though these models may still be suitable for transdermal delivery.
Skin appendages
-
- Sweat glands
Human skin contains three types of sweat glands: eccrine, apocrine, and apoeccrine glands. Eccrine glands are the most abundant and distributed across the whole body, responsible for the majority of sweat excretion. Apocrine and apoeccrine glands, on the other hand, are localized to specific areas, primarily in the axillary (underarm) regions. The primary function of all sweat glands is to aid in thermoregulation, helping to maintain optimal body temperature.
-
- Sebaceous glands
In the human body, sebaceous glands are associated with hair follicles. These holocrine glands secrete sebum, an oily substance believed to play a role in the skin’s defense. While the full extent of their importance remains unclear, sebum is thought to possess antibacterial and antifungal properties, contributing to the skin’s protective barrier.
-
- Hair follicles
The hair follicle is essential for thermoregulation, distributing sweat and sebum, enabling sensory and tactile functions, and supporting skin regeneration and repigmentation.
Currently, the integration of hair follicles, sweat, or sebaceous glands in reconstructed or bioprinted skin models remains limited, with technology still under development4. As a result, the lack of fully functional skin appendages in these models restricts their ability to accurately replicate all functions of human skin, making them unsuitable for testing hair care products or those targeting sebum production.
Peripheral nerves
The skin is a very sensitive organ densely innervated by a meshwork of peripheral nerves defined by the presence of specific receptors and involved in the perception of tactile mechanical stimuli and of noxious stimuli (e.g. pain, high temperature or irritant).
Free peripheral nerve endings can be found in the dermis (e.g. surround hair follicles) as well as deep in the epidermis. For example, motor nerves are sympathetic nerves located in the dermis that secrete neurotransmitters, neuromodulators, and neuropeptides to regulate sweat gland secretion, vasoconstriction, and temperature balance adjustment. Nociceptive sensory neurons (nociceptors) project both in the dermis and epidermis. Like resident immune cells, nociceptors represent a unique type of “skin‐resident cell” capable of responding to harmful stimuli from the microenvironment and regulating inflammatory response. Recently, skin-projecting nociceptors have been shown to interact with skin-resident immune cells (e.g. DC and mast cells) to regulate immune defenses against invading pathogens but also inflammation, pain and itch.
Obviously, only cut off nerve endings remain in skin explants because of the surgery procedure. Nevertheless, several researchers have demonstrated the possibility of innervating reconstructed human skin models using iPS5 bringing hope to better understand the interaction of innervation and skin homeostasis.
Blood circulation
The skin is a highly vascularized organ, with blood and lymphatic vessels concentrated in the dermis and hypodermis. Interestingly, the epidermis lacks a vascular system. Circulation within the skin plays a crucial role in thermoregulation, nutrient delivery, and overall skin health. This intricate network of blood vessels and capillaries supplies oxygenated blood and essential nutrients to maintain skin function. However, replicating the skin’s complex vasculature in vitro remains a significant challenge.
While no fully reconstructed skin models can yet replicate human skin circulation, there have been some advancements in developing skin models with vascular components. Many studies report the alignment of endothelial cells into vessel-like structures when these cells are added to the dermal layer of a reconstructed in vitro skin model6,7,8.
Another promising approach involves microfluidic-based skin-on-chip models, which use microfluidic channels to simulate blood flow beneath a layer of cultured skin cells. Though not a complete circulatory system, they allow for nutrient delivery and waste removal.
Finally, some researchers are also exploring 3D bioprinting techniques to create skin models with embedded vascular-like channels. These channels can be perfused with culture media, mimicking blood flow in a controlled environment.
Melanocytes and melanogenesis
In native human skin, melanocytes are naturally present, with their density varying with the body site: from about 900 melanocytes per square millimeter on the back to approximately 1,500 melanocytes per square millimeter in the genital region9. Interestingly, melanocyte density shows minimal variation across individuals of different backgrounds10,11. Melanocytes are also found in hair follicles, where they contribute to hair pigmentation.
Melanogenesis, the complex biochemical process by which melanin is synthesized within melanocytes, takes place in specialized organelles called melanosomes. This process is crucial in determining skin color and providing essential protection against UV radiation.
Basic reconstructed skin models lack melanocytes. However, more advanced models now include pigmentation to better represent the diversity of human skin and allow for the study of pigmentation-related processes. These pigmented models are created by incorporating melanocytes into the epidermal layer of reconstructed skin. While they are a significant advancement, these models may not fully capture all aspects of natural human skin pigmentation, as the complexity of the pigmentation process and individual variations remain challenging to replicate entirely in vitro.
The presence of functional skin resident immune cells
Skin-resident immune cells are essential for maintaining skin homeostasis and providing defense against pathogens. Most of the skin’s immune system resides in the epidermis and dermis, though immune cells, like macrophages, are also found in the hypodermis. The epidermis contains keratinocytes, Langerhans cells (LCs), and CD8+ T cells, while the dermis hosts macrophages, various subsets of dermal dendritic cells (DCs), mast cells, and lymphocytes including T cells, natural killer (NK) cells, and B cells. These cells provide continuous monitoring of the skin environment, ready to respond to infections or injuries.
Some advanced reconstructed human skin models now include immune cells. In these models, a 3D co-culture of keratinocytes (representing the epidermis) with immune cells like dendritic cells or T cells provides an immune component. Full-thickness skin equivalents may incorporate immune cells like LCs, DCs, T cells, and macrophages, while skin-on-a-chip models can also include immune cells and allow for their migration.
These immune-competent skin models are used to study skin immune responses, inflammatory skin diseases (such as atopic dermatitis and psoriasis), and drug testing. Although they mark a significant advancement, fully replicating the complexity of the skin’s immune environment remains challenging.
Researchers are continually working to improve the physiological relevance and complexity of these models.
Finally, in addition to defending the host against damaging or potentially damaging environmental cues, the immune system plays a critical role in maintaining skin homeostasis. Through interactions with other skin cells, it adapts to environmental changes. These interactions are currently absent in reconstructed skin models.
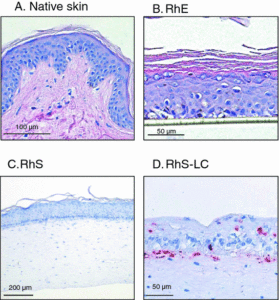
Figure 3. Histology (hematoxylin–eosin staining) of (a) native skin (biopsy), (b) reconstructed human epidermis (RhE) on a polycarbonate filter, (c) reconstructed epidermis on fibroblast-populated dermis (RhS), and (d) An HLA-DR immunohistochemical staining of MUTZ-LC integrated in reconstructed epidermis on fibroblast-populated collagen hydrogel (RhS-LC) (stratum corneum was lost during immunohistochemical staining procedure). Modified from (18).
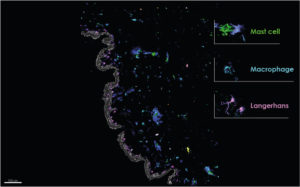
NativeSkin® multiplex imaging. Genoskin.
Skin barrier function
Human skin serves as a protective barrier, regulating the penetration of water, nutrients, ions, and environmental stimuli. It shields the body from chemical exposure, ultraviolet radiation, pathogens, and dehydration while maintaining tissue homeostasis.
The skin barrier function is composed of four key components:
-
- Physical barrier: This first line of defense includes the stratum corneum and tight junctions. The stratum-corneum acts as an “outside-in” barrier, preventing the penetration of external elements. Tight junctions form an “inside-out” barrier to protect against dehydration.
In vitro 3D skin models are commonly used to study this physical barrier, as they can form a stratified epidermis with stratum corneum and tight junctions. However, these models often show differences in lipid composition and organization, leading to increased permeability13. - Chemical barrier: This barrier consists of defense molecules like antimicrobial peptides and reactive oxygen species (ROS). While it plays a key role in defending against pathogens, it remains less studied in 3D skin models, leaving room for optimization.
- Immunological barrier: Acting as a secondary defense when the physical barrier is compromised, the immunological barrier includes all skin resident immune cells. The interplay between keratinocytes and these immune cells is essential for tissue homeostasis and inflammatory processes. Immunological barriers are difficult to study in static in vitro systems, and no reconstructed model contains all the immune cells found naturally in the skin.
- Microbial barrier: This component, the skin microbiome, maintains an acidic pH to inhibit bacterial growth. While many studies now focus on the skin microbiome, including efforts to seed whole patient-derived microbiomes on reconstructed models, the complex host-microbe and microbe-microbe interactions on human skin are still not fully understood.
- Physical barrier: This first line of defense includes the stratum corneum and tight junctions. The stratum-corneum acts as an “outside-in” barrier, preventing the penetration of external elements. Tight junctions form an “inside-out” barrier to protect against dehydration.
Microbiome
The human skin microbiome is a complex ecosystem of microorganisms, including bacteria, fungi, viruses, and archaea, that inhabit the skin. The skin microbiome protects against pathogens by competing for resources and attachment sites, as well as producing antimicrobial compounds. Commensal bacteria, for instance, produce free fatty acids that help maintain the skin’s acidic pH. Additionally, the microbiome interacts with keratinocytes and immune cells, helping to calibrate immune defenses.
Efforts have been made to incorporate microbiome elements into reconstructed human skin models, but fully replicating the native skin microbiome remains challenging. Most basic reconstructed skin models are initially sterile and lack microbes by default. Selective colonization is possible, allowing researchers to introduce specific microorganisms or strains. Some commercially available models are designed to host microflora, either with specific strains or commensal skin microbiota. However, even with microbial communities applied, replicating the intricate host-microbe and microbe-microbe interactions of human skin is complex. Furthermore, the short lifespan of most models limits studies involving slower-growing microbes or requiring long-term stability.
Conclusions
While significant progress has been made, challenges remain. Reconstructed human skin models offer a more relevant and ethical alternative to animal skin; however, they still lack key features such as skin appendages, a fully functional immune system, and hypodermis. These limitations currently prevent their use in efficacy and safety testing of non-topical compounds, which is essential for a large number of drugs on the market today.
Ex vivo human skin samples, on the other hand, can be kept alive outside of the human body for several days, providing a robust platform for toxicity and efficacy testing of pharmaceuticals, chemicals, and cosmetics. These models yield more relevant and comprehensive data, making real human skin models the gold standard for delivering the most accurate and reliable results.
References
- Nakamura, M, Haarmann‐Stemmann, T, Krutmann, J, Morita, A. Alternative test models for skin ageing research. Exp Dermatol. 2018; 27: 495– 500.
- Sheasgreen J, Klausner M, Kandárová H, Ingalls D. The MatTek story – how the three Rs principles led to 3-D tissue success! Altern Lab Anim. 2009 Dec;37(6):611-22.
- Bataillon M, Lelièvre D, Chapuis A, et al. Characterization of a New Reconstructed Full Thickness Skin Model, T-Skin™, and its Application for Investigations of Anti-Aging Compounds. Int J Mol Sci. 2019;20(9):2240. Published 2019 May 7.
- Randall MJ, Jüngel A, Rimann M, Wuertz-Kozak K. Advances in the Biofabrication of 3D Skin in vitro: Healthy and Pathological Models. Front Bioeng Biotechnol. 2018 Oct 31;6:154.
- Muller Q, Beaudet MJ, De Serres-Bérard T, Bellenfant S, Flacher V, Berthod F. Development of an innervated tissue-engineered skin with human sensory neurons and Schwann cells differentiated from iPS cells. Acta Biomater. 2018 Dec;82:93-101.
- Lei XH, Ning LN, Cao YJ, Liu S, Zhang SB, Qiu ZF, Hu HM, Zhang HS, Liu S, Duan EK. NASA-approved rotary bioreactor enhances proliferation of human epidermal stem cells and supports formation of 3D epidermis-like structure. PLoS One. 2011;6(11):e26603.
- Montano, I., Schiestl, C., Schneider, J. et al. (2010). Formation of human capillaries in vitro: The engineering of prevascular-ized matrices. Tissue Eng Part A 16, 269-282.
- Tremblay, P. L., Hudon, V., Berthod, F. et al. (2005). Inoscu-lation of tissue-engineered capillaries with the host’s vas-culature in a reconstructed skin transplanted on mice. Am J Transplant 5, 1002-1010.
- Jorizzo JL, Bolognia JL, Rapini RP. Dermatology: 2-Volume Set: MOSBY. 2008. (ELSEVIER)
- Alaluf S, Atkins D, Barrett K, Blount M, Carter N, et al. Ethnic variation in melanin content and composition in photoexposed and photoprotected human skin. Pigment Cell Res. 2002;15:112–118.
- Tadokoro T, Yamaguchi Y, Batzer J, Coelho SG, Zmudzka BZ, et al. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J Invest Dermatol. 2005;124:1326–1332.
- Rodrigues Neves C, Gibbs S. Progress on Reconstructed Human Skin Models for Allergy Research and Identifying Contact Sensitizers. Curr Top Microbiol Immunol. 2018 Jun 23.
- Niehues, H, Bouwstra, JA, Ghalbzouri, AE, Brandner, JM, Zeeuwen, PLJM, van den Bogaard, EH. 3D skin models for 3R research: The potential of 3D reconstructed skin models to study skin barrier function. Exp Dermatol. 2018; 27: 501‐ 511.
Comments are closed.