Why testing & mitigating Injection Site Reactions is critical during drugs/vaccines development?

Injection site reactions (ISR) are a common occurrence when a person receives an injection in the skin, such as a vaccine or self-administered biologics.
These reactions occur due to the activation of the immune system at the injection site.
When a foreign substance, such as a vaccine or medication, is injected into the body, the immune system recognizes it as an invader and begins to mount a defense. This defense can include the release of inflammatory molecules, such as cytokines and chemokines, and the recruitment of immune cells to the site of the injection.
These immune responses can result in ISRs, which can include redness, swelling, pain, and warmth at the site of the injection1, 2, 3. These reactions are generally mild and short-lived, and most people recover without any complications.
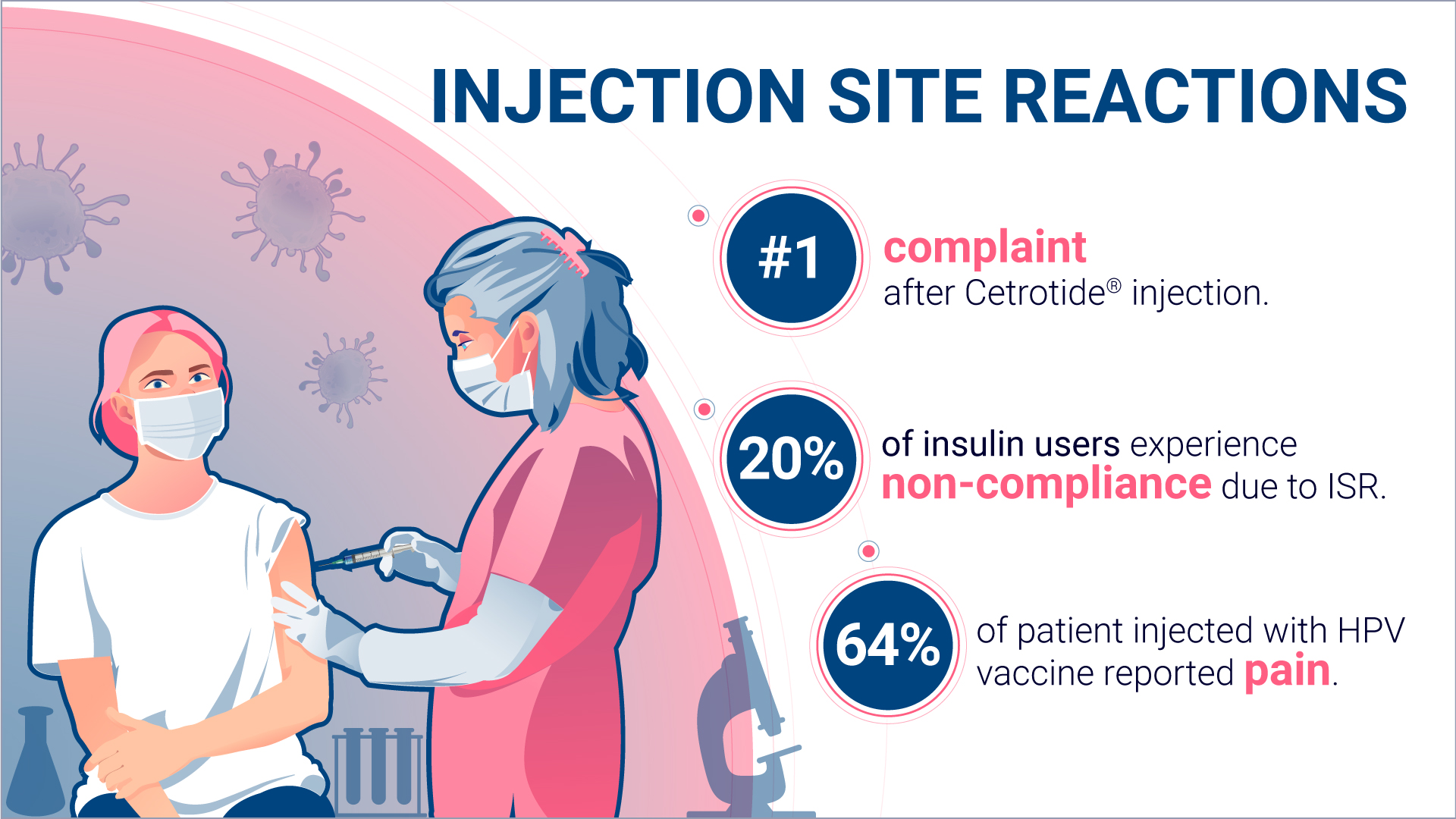
It is well recognized that ISRs are a common side effect associated with many drugs, particularly those that are administered subcutaneously or intramuscularly, like anakinra4, cetrorelix5, and adalimumab6. In addition to these drugs mentioned, some examples of other drugs that have been associated with ISRs include insulin7, growth hormone8, and etanercept9.
In some cases, however, ISRs can be more severe, and may require medical attention. These can include large swelling, redness, tissue necrosis, abscess formation, ulceration, severe pain, or allergic reactions. It’s particularly the case with administration of medication such as Influenza, Rabies, Yellow fever and Pertussis vaccine10, as well as Interferon11.
In addition, ISRs may also lead to anxiety or fear about receiving subsequent injections, which contribute to non-compliance. For example, a patient may become reluctant to take medication or avoid future injections altogether out of fear of experiencing a reaction again.
Some examples include:
Insulin injections:
Insulin was the first biologic ever developed, and was reported to be used by 7.9 millions of American with diabetes12 in 2021.
Injection site reactions are a common side effect of insulin injections, and can include redness, swelling, and pain at the injection site. Severe reactions, such as abscess formation, can occur in rare cases.
A study called “Correlates of insulin injection omission”, published in 2010 in Diabetes Care, assessed factors associated with patient frequency of intentionally skipping insulin injections. Results shown that 20% of the 502 adults included in the study would intentionally omit to take their insulin, one of the reason being injection pain13.
Not complying with insulin therapy, usually leads to poor blood sugar control and potential permanent complications such as retinopathy, neuropathy, and cardiovascular disease.
Human papillomavirus (HPV) vaccine14:
Pain and discomfort at the time of injection or soon after is a common thought when talking about HPV vaccine.
In 2021, the study “How much will it hurt? HPV vaccine side effects and influence on completion of the three-dose regimen15 reported that 64% of parents of a daughter receiving a dose of HPV vaccine were complaining about pain. Although the study concluded that pain from HPV vaccine was equivalent to that from other adolescent vaccines, such reactions lead to vaccine hesitancy and decreased compliance with the vaccine schedule.
In 2015, the study “Safety of human papillomavirus vaccines: a review” , published in Expert Opinion on Drug Safety reported that: “public concern and rumors about adverse events (AE) represent an important barrier to overcome in order to increase vaccine coverage. Passive surveillance of AEs is an important tool for detecting safety signals, but it should be complemented by activities aimed at assessing the real cause of all suspect AEs.”
Methotrexate injections16:
Methotrexate is a small molecule used in various autoimmune conditions. A paper published in May 2022 reviewed a case of injection site reaction following subcutaneous injection of methotrexate. While rare in that case, those adverse events can, here again, lead to discontinuation of treatment.
As a last example, let’s talk about cetrorelix:
Cetrorelix, authorized as Cetrotide® by the European Medicines Agency in 199917 and by the Food and Drug Administration in 200018 is gonadotropin-releasing hormone (GnRH) antagonist, used to prevent ovulation in women going through fertility procedures.
The second most common side effect reported are “reactions at the injection site, such as redness, swelling and itching”19.
(Read our case study here.)
Finally, it is well-understood that medication non-compliance, including that due to ISRs, has significant economic and health consequences for both patients and the healthcare system as a whole. These complications can result in increased healthcare costs, decreased quality of life, and increased morbidity and mortality.
Our solutions:
Genoskin provides valuable expertise to pharmaceutical and biotechnology companies by characterizing, before clinical trials, human immune responses to their drug candidates or other biological agents in bio-stabilized and immunocompetent human skin platforms (HypoSkin human skin models). This approach significantly reduces the risk of unexpected adverse events and accelerates the drug development process.
Genoskin’s ISR Platform® is an ideal tool for non-clinical testing, enabling drug candidates to be assessed before proceeding to clinical phases. By using this platform, pharmaceutical and biotechnology companies can also identify and understand local adverse reactions and take steps to mitigate any safety concerns that arise during clinical trials.
Overall, Genoskin’s human skin models and ISR Platform® offer a powerful combination of tools that can help to speed up drug development, improve safety and efficacy testing, and ultimately bring safer and more effective drugs to market.
Let’s work together to develop safer drugs.
References
1Otani IM, Levin AS, Banerji A. Cutaneous Manifestations of Reactions to Biologics. Curr Allergy Asthma Rep. 2018 Feb 21;18(2):12.
2Patel SV, Khan DA. Adverse Reactions to Biologic Therapy. Immunol Allergy Clin North Am. 2017 May;37(2):397-412.
3Thomaidou E, Ramot Y. Injection site reactions with the use of biological agents. Dermatol Ther. 2019 Mar;32(2)
4Kaiser C, Knight A, Nordström D, Pettersson T, Fransson J, Florin-Robertsson E, Pilström B. Injection-site reactions upon Kineret (anakinra) administration: experiences and explanations. Rheumatol Int. 2012 Feb;32(2):295-9.
5Verschraegen CF, Westphalen S, Hu W, Loyer E, Kudelka A, Völker P, Kavanagh J, Steger M, Schulz KD, Emons G. Phase II study of cetrorelix, a luteinizing hormone-releasing hormone antagonist in patients with platinum-resistant ovarian cancer. Gynecol Oncol. 2003 Sep;90(3):552-9.
6Scheinfeld N. Adalimumab: a review of side effects. Expert Opin Drug Saf. 2005 Jul;4(4):637-41.
7Müller CSL, Bourg C, Schweitzer LF, Friesenhahn-Ochs B, Pföhler C. Injection Site Reaction to Various Insulins as Type III Allergy With Urticarial Vasculitis in a Patient With Type I Diabetes Mellitus. Am J Dermatopathol. 2023 Feb 1;45(2):86-89.
8Manavela MP, Danilowicz K, Bruno OD. Skin reaction and fever after treatment with pegvisomant in a patient with acromegaly. Clin Ther. 2010 Feb;32(2):246-9.
9Combe B. Update on the use of etanercept across a spectrum of rheumatoid disorders. Biologics. 2008 Jun;2(2):165-73.
10Hervé C, Laupèze B, Del Giudice G, Didierlaurent AM, Tavares Da Silva F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019 Sep 24;4:39.
11Maurelli M, Bergamaschi R, Antonini A, Fargnoli MC, Puma E, Mallucci G, Totaro R, Girolomoni G. Interferon-beta injection site reactions in patients with multiple sclerosis. J Dermatolog Treat. 2018 Dec;29(8):831-834.
12“Fewer diabetes patients are picking up their insulin prescriptions – another way the pandemic has delayed health care for many” 11/18/2021 – Ismaeel Yunusa – University of South Carolina.
13Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care. 2010 Feb;33(2):240-5.
14Stillo M, Carrillo Santisteve P, Lopalco PL. Safety of human papillomavirus vaccines: a review. Expert Opin Drug Saf. 2015 May;14(5):697-712.
15Reiter, Paul L et al. “How much will it hurt? HPV vaccine side effects and influence on completion of the three-dose regimen.” Vaccine vol. 27,49 (2009): 6840-4.
16https://medlineplus.gov/druginfo/meds/a603002.html
17https://www.ema.europa.eu/en/medicines/human/EPAR/cetrotide
18https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21-197_Cetrotide.cfm
19https://www.ema.europa.eu/en/documents/overview/cetrotide-epar-medicine-overview_en.pdf
Comments are closed.





