Immunogenicity Series
Part 1 – Defining immunogenicity
Immunogenicity plays a pivotal role in drug and vaccine development and is essential for optimizing therapeutic efficacy and safety
by understanding the immune system’s response to the compounds being tested.
This is particularly crucial in the era of biologics and personalized medicine, where the fine balance between therapeutic benefit and immune reactivity is critical. Whether it’s the development of novel vaccines or the design of therapeutic proteins, the assessment of immunogenicity guides researchers and clinicians in optimizing treatment outcomes and ensuring patient safety.
Immunogenicity and drug development
Definition
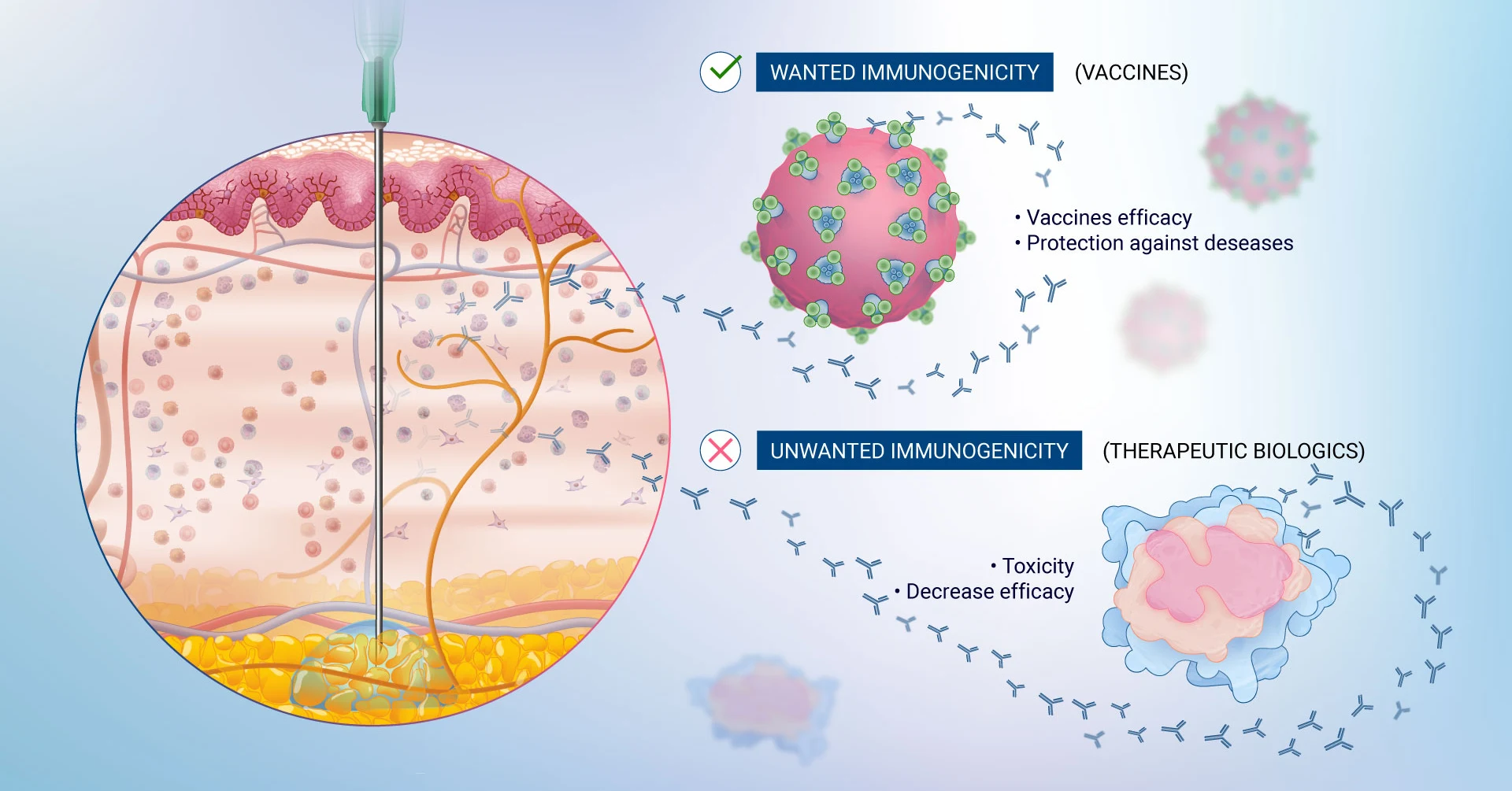
Immunogenicity is defined as the inherent ability of a substance, such as a drug or vaccine, to elicit an immune response within the body. Immunogenicity is a pivotal concept in drug development as the immune response can range from beneficial (as with vaccines) to potentially adverse and unwanted (as in the case with therapeutic proteins). In the latter, immunogenicity can be detrimental and lead to the development of anti-drug antibodies (ADAs) that neutralize the effectiveness of a therapeutic drug or cause unwanted side effects. Immunogenicity is a critical consideration in the development and approval of biologic drugs, as it directly impacts their efficacy and safety1.
Immunogenicity is frequently associated with reactogenicity. Reactogenicity is specifically related to the immediate reaction or side effects that occur following vaccination or drug administration. These reactions are typically short-term and can include symptoms like redness, swelling, pain at the injection site, fever, or muscle aches. Reactogenicity is a measure of the physical manifestation of the body’s inflammatory response to the vaccine or drug2. In the case of vaccines, while it can be uncomfortable, it is often a sign that the body is building protection against the disease.
Why is it important? Immunogenicity: A crucial aspect of drug and vaccine development
The immune system, our body’s defense mechanism, is responsible for identifying and neutralizing foreign invaders, including viruses, bacteria, and parasites. However, it can also detect and respond to therapeutic agents, potentially compromising their efficacy and causing unwanted adverse reactions.
The study of immunogenicity is a cornerstone in the development of drugs and vaccines, serving as a critical factor in determining their safety and efficacy. In the realm of biopharmaceuticals, particularly those involving therapeutic proteins, antibodies, and vaccines, understanding and managing immunogenicity is pivotal. The immune system’s response to these biologics can significantly impact their therapeutic effectiveness and patient safety.
- Safety and Efficacy: Immunogenicity can lead to the production of ADAs, which can neutralize the therapeutic effects of the compound or cause adverse immune reactions. In the case of vaccines, a robust immunogenic response is desired to provide protection against the target disease. Therefore, assessing immunogenicity helps in predicting and enhancing their clinical performance.
- Regulatory Compliance: Regulatory agencies like the FDA and EMA have set guidelines for immunogenicity assessment, making it a mandatory aspect of the drug and vaccine approval process. This assessment ensures that the benefits of the therapeutic outweigh its potential immunogenic risks to patients.
- Personalized Medicine: Understanding the immunogenic potential of drugs aids in the development of personalized medicine strategies. It allows for the prediction of patient-specific responses to biologics, leading to more tailored and efficacious treatments.
- Advancements in Drug Design: Studying immunogenicity guides scientists in modifying the molecular structure of biologics to diminish their immunogenic potential while preserving their therapeutic efficacy. This is particularly relevant in the development of biosimilars and next-generation biologics3.
Understanding immunogenicity
Factors impacting immunogenicity
Immunogenicity is influenced by a myriad of factors that can be broadly categorized into three groups: product-related, patient-related, and treatment-related factors4.
- Product-Related Factors: The inherent properties of biologic drugs significantly influence their potential to provoke an immune response. This includes the molecular structure, presence of impurities or aggregates5, post-translational modifications, and the degree of similarity to endogenous proteins. For instance, non-human sequences in therapeutic proteins can be highly immunogenic as they can prompt the immune system to elicit a response against a foreign element in the body. Additionally, the formulation and stability of the product can also impact its immunogenicity.
- Patient-Related Factors: Individual patient characteristics significantly influence how the immune system responds to biologics. Genetic factors, such as HLA type, can predispose individuals to different immunogenic responses. Concurrent conditions, like autoimmune diseases, and the patient’s immune status, whether immunocompromised or immunosensitive, also play a role. Previous exposure to similar drugs can lead to cross-reactivity and heightened immune responses. Therefore, considering individual patient characteristics is crucial for tailoring and optimizing the use of biologics to ensure desired therapeutic outcomes while minimizing potential risks.
- Treatment-Related Factors: The route of administration (intravenous, subcutaneous, etc.)6, dosage, frequency of administration, and duration of treatment can affect the immunogenicity of a drug. For example, subcutaneous administration may lead to higher immunogenicity compared to intravenous administration due to increased exposure to the immune system as the way a drug is administered can affect its absorption, distribution, and interaction with the immune system. The dosage, frequency, and duration of treatment determine the exposure of the immune system to the drug over time, impacting the likelihood and intensity of immune responses. Therefore, careful consideration of these parameters is essential in drug development to optimize therapeutic outcomes and minimize potential immunogenic risks.
Understanding these factors is crucial for predicting and managing the immunogenicity of biopharmaceuticals. The development and use of accurate human models to predict immunogenicity early on in the drug development process then becomes essential, ultimately leading to safer and more effective therapies.
Types of immune responses
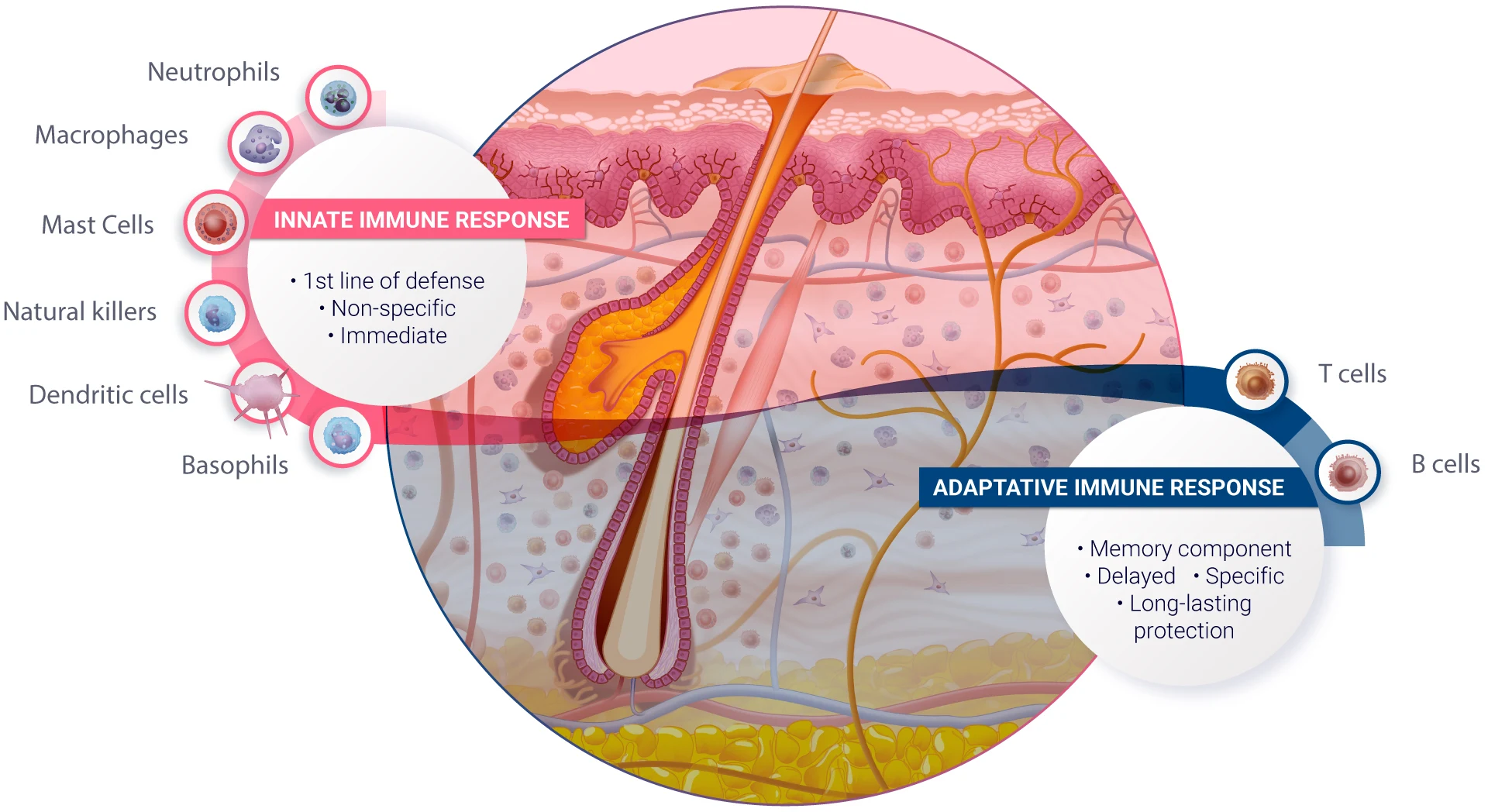
The immune system exhibits diverse responses to foreign substances, including drug compounds. These responses can be broadly classified into two main types: innate and adaptive immune responses. Both play crucial roles in defending the body against pathogens and foreign substances, but they differ in their mechanisms, timing, and specificity.
- Innate Immune Response: The innate immune response is the body’s first line of defense to foreign substances. This arm of the immune system lacks specificity and can therefore initiate an immediate response, occurring within hours of exposure to a foreign substance. It involves physical barriers like skin and mucous membranes, cellular components like phagocytic cells (e.g., neutrophils, macrophages), natural killer cells, and various proteins including the complement system. These multiple components together promote an inflammatory response and enhance phagocytosis and cell-mediated killing, thus contributing to the destruction of foreign substances7.
Certain drug compounds can trigger the innate immune system. For example, some vaccine adjuvants are designed to activate the innate immune response to enhance the vaccine’s effectiveness. Additionally, some drug formulations can inadvertently activate innate immune components, leading to inflammation or other unwanted side effects. - Adaptive Immune Response: The adaptive immune response exhibits remarkable specificity towards specific pathogens or foreign substances. While its development is much slower compared to the innate immune response, taking days to weeks to achieve full effectiveness, it incorporates a memory component that allows for long-lasting protection upon re-infection. The adaptive immune system consists of lymphocytes, including B cells and T cells. B cells produce antibodies that specifically target antigens expressed on foreign agents, while T cells can directly kill infected cells (CD8+ cytotoxic T cells), help regulate other immune cells (CD4+ Helper T cells) or help maintain immune system balance by suppressing excessive immune responses and preventing autoimmune reactions (Regulatory T cells, Tregs).
In the context of drug compounds, the adaptive immune response is critical in the development of immunogenicity, for instance, therapeutic proteins can be recognized as foreign by the adaptive immune system, leading to an immune response against the drug. This immune response can lead to the formation of ADAs which can bind to the therapeutic protein and directly impair or abrogate its function leading to a reduced efficacy and the need to dose patients more frequently. ADAs can also lead to severe adverse events by binding to the endogenous counterpart of the therapeutic agent, for instance, recombinant human thrombopoietin has been reported to induce the generation of ADAs leading to thrombocytopenia8.
Concluding Remark
In conclusion, the exploration of immunogenicity in drug and vaccine development is critical to ensure the safety and effectiveness of new therapeutics. This complex interplay between biologics and the immune system underscores the importance of meticulous research and development in the pharmaceutical industry. By understanding the factors that influence immunogenicity and the nature of immune responses, scientists and clinicians can better predict, manage, and mitigate potential risks associated with biopharmaceuticals. This knowledge is instrumental in the creation of safer, more effective therapeutic agents, and in ensuring regulatory compliance. As we continue to navigate the challenges and opportunities in biopharmaceutical development, the study of immunogenicity remains a beacon, guiding us toward innovative solutions to revolutionize patient care.
References
1De Groot AS et al. “Immunogenicity of protein therapeutics.” Trends Immunol. 2007 Nov;28(11):482-90.
2Hervé, C. et al. “The how’s and what’s of vaccine reactogenicity.” npj Vaccines 4, 39 (2019)
3Doevendans E et al. “Immunogenicity of Innovative and Biosimilar Monoclonal Antibodies.” Antibodies (Basel). 2019 Mar 5;8(1):21
4Fernandez Rodriguez et al. “Immunogenicity in Protein and Peptide Based-Therapeutics: An Overview.” Current Protein & Peptide Science, 2018;19(10):958-971
5Ratanji, K. D. et al. “Immunogenicity of therapeutic proteins: Influence of aggregation.” Journal of Immunotoxicology, 2014;11(2):99-109
6Jarvi NL et al. “Immunogenicity Challenges Associated with Subcutaneous Delivery of Therapeutic Proteins.” BioDrugs. 2021 Mar;35(2):125-146
7Alberts B, Johnson A, Lewis J, et al. “Molecular Biology of the Cell.” 4th edition. New York: Garland Science; 2002. Innate Immunity.
8Kuriakose A et al. “Immunogenicity of Biotherapeutics: Causes and Association with Posttranslational Modifications.” J Immunol Res. 2016;2016:1298473.
Comments are closed.