Better science through ethical sourcing
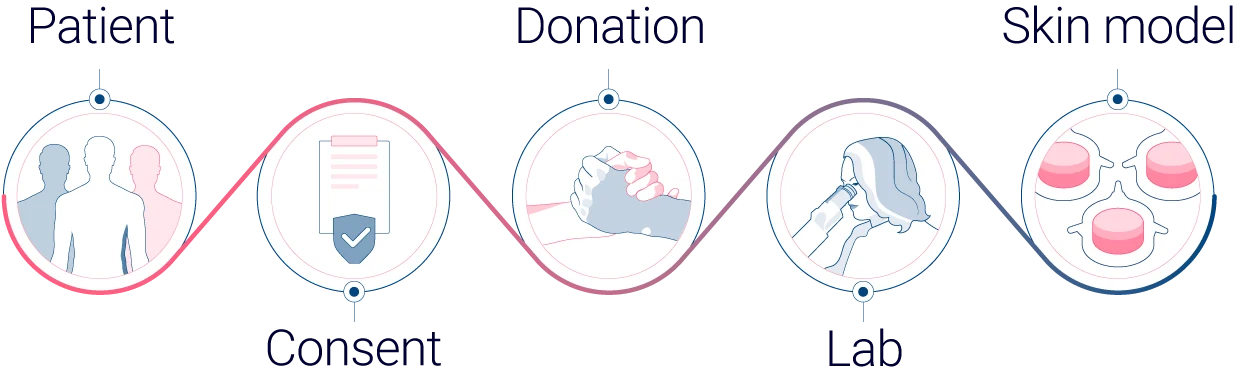
From donated human skin to innovative human skin models

Human skin tissue is classified as a biological sample, and its collection and use must comply with strict legal and ethical requirements. To ensure this, Genoskin works exclusively with hospitals and clinics under secure Biological Sample Transfer contracts that safeguard both donor confidentiality and research integrity. These contracts mandate that each sample is collected following rigorous protocols and accompanied by anonymized donor information allowing us to maintain full traceability:
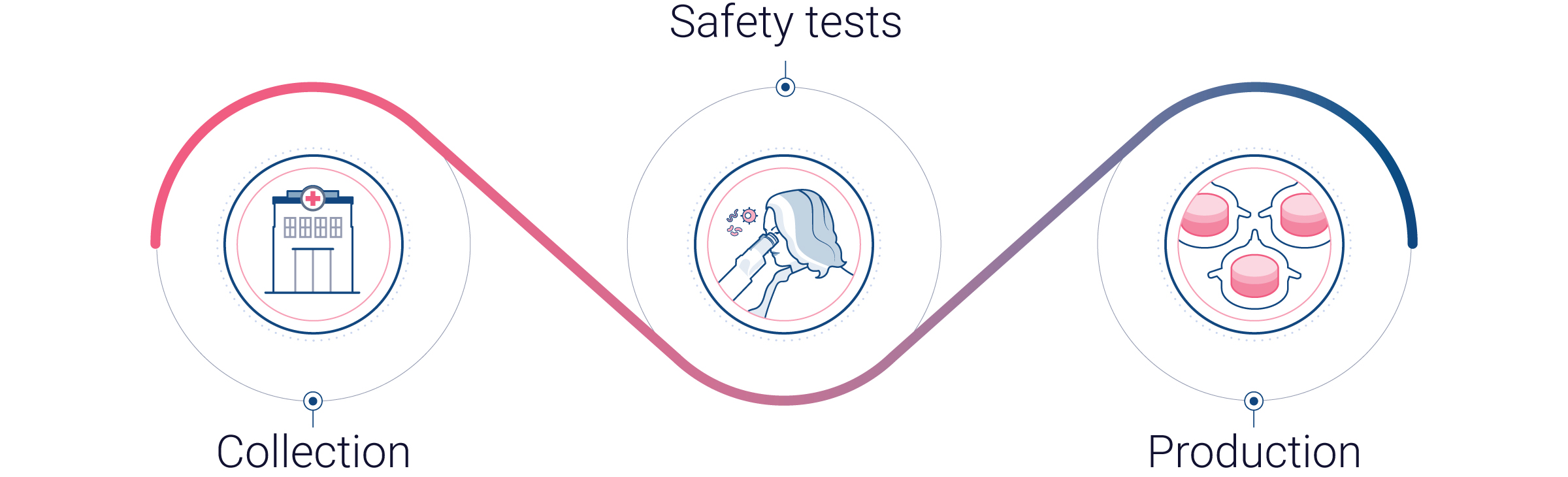
Collection
Our human skin samples are collected right after surgery and immediately transported to Genoskin’s facilities.
Safety tests
Before using a skin sample, our skin experts conduct safety tests for Hepatitis B and C, as well as HIV-1 and HIV-2. Only negative samples are used to produce our skin models.
Production
Production of human skin models occurs within 24 hours after surgery. In the interval between donor surgery and model production, the skin samples are maintained at optimal temperature.
Legal guidelines on the use of donated human skin tissue

Animal experiments have been widely used to assess toxicity and safety and to support the development of new pharmaceutical, chemical or cosmetic products. However, increasing awareness of the fate of laboratory animals has encouraged lawmakers to restrict the use of animals during testing procedures:
Legal & ethical compliance
Genoskin fully complies with ethical and legal standards:
Human data to de-risk drug development

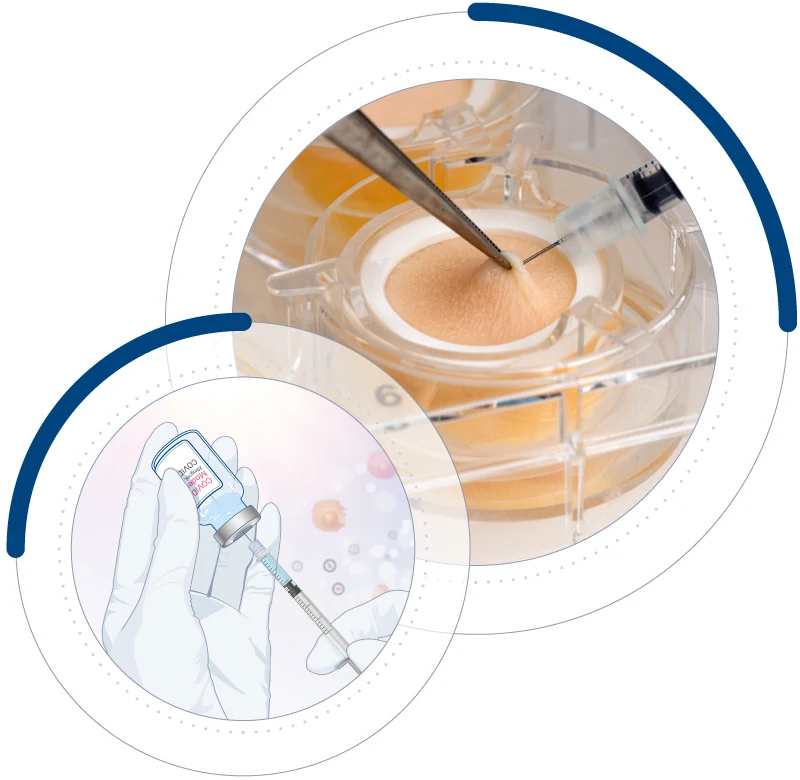
At Genoskin, we provide innovative, animal-component-free (ACF) / xeno-free (XF) human skin models as reliable alternatives to animal testing. Instead of being discarded, the collected human skin is transformed into scientifically valuable tools that:
Our models support the biopharmaceutical, chemical, & cosmetic industries in developing safe and effective products, while actively reducing animal testing.
Our approach aims to improve drug development by generating relevant human data instead of relying on animal models. By using human skin tissue, our test platforms assess the immunogenicity and immunotoxicity of drugs and vaccines across different administration routes before clinical trials. This strategy provides more predictive results, helping science and industry move forward responsibly.
Certified quality processes
Genoskin’s activities are divided into processes and monitored by demanding performance indicators

A robust documentary system oversees all of the company’s activities. Regular internal audits are carried out in parallel with external audits to ensure the proper application of our standard operating procedures and to maintain continuous improvement. The company is audited by AFNOR, an external certification body, yearly. All processes are verified (Sourcing/Biobanking, Production, Services, R&D, Sales, Quality, etc.). These audits are organized over a 3-year cycle: 1 initial audit and 2 follow-up audits.





