Human skin to better predict clinical trial outcomes
Based on the Tech Nation interview with Dr. Eric Merle, our Chief Bussiness Innovation Officer by Moira Gunn, in February 2022. The entire Tech Nation Radio Podcast is available here. Eric’s interview starts at 44:00 mins.
Close is not good enough
We’ve grown used to testing drugs in animals because some animals are believed to be close enough to humans. However, there are plenty of diseases for which no animal models are available. Moreover, whether a disease is rare or common, reproducing a certain condition in an animal model takes a tremendous amount of work. Instead of trying to reproduce something in an animal model, why not leverage what already exists? Wouldn’t it be more relevant to use a human model during preclinical trials? Why not use human skin?

The advantages of using human skin in preclinical trials
Animal testing is on its way out
Just before the COVID pandemic, the US Environmental Protection Agency (EPA) declared that 2035 would mark the end of the agency’s funding for testing on mammals for research. The EPA has been awarding millions of dollars to seek out alternatives. Similarly, the US Department of Veterans Affairs will restrict funding for experiments on dogs and “end or reduce” experiments on dogs, cats and primates by 2025. Even the US Department of Agriculture has banned research using cats since 2019. The trend is clear: the goal is for animal testing to be retired. It’s what the general public wants. It’s a growing request from regulatory bodies. The pharmaceutical and biotech industry need to do better than animal testing. It doesn’t sufficiently demonstrate whether a drug will work nor how well it will work when it moves into the clinical phases.

``Even in the best research, an animal remains an animal.``
Eric Merle – Genoskin Chief Business Innovation OfficerBetter tools to predict clinical trial outcomes
Human skin provides a solution. Having the ability to obtain human data, before testing in humans during clinical trials, not only makes the drug development process less risky, it may improve the number of drugs that will be developed and our understanding of disease pathways to tackle the problem.

Human skin also helps solve a wide range of related issues:
- It takes 12 to 15 years to get from the lab bench to an approved drug.
- There’s a 90% failure rate. Only one out of 10 drug development projects succeeds.
- Recruiting patients in a clinical trial is a long and complicated process.
- Reproducing population diversity and finding patients that are of different age groups and of different ethnic backgrounds can be very time-consuming.
- Preclinical trials currently focus on animal models and – often – human cell cultures but:
- they don’t reproduce the 3-D environment of human organs
- they don’t have the metabolism of a normal human organ.
Functional human skin to increase clinical trial success
Clinical trials often only reflect the end results of a trial. However, these results are merely the tip of the iceberg. Genoskin is a research partner: we’re here to help you unravel the different pathways of your drug. Understanding the biomarkers of the effects of a particular drug is key to accelerating the speed at which it can be developed. Our experts use live human skin to answer questions from pharmaceutical and biotech companies and predict drug effects and side-effects. We uncover what’s inside that black box to increase clinical trial success rates.

Genoskin’s human skin models provide the opportunity to conduct experiments with topical, systemic, subcutaneous and intradermal drugs on live human skin and immune cells in their natural environment.
- On average, the preclinical stage of drug development takes five to seven years. Using the human data available in human skin, that time span can be compressed, because … it’s real human data.
- Even though it requires the legal authorization of an IRB ethical review board, human skin is available. Of course, it’s not possible to just start working on human tissue. This also requires the patient’s consent but a lot of patients are ready to donate leftover skin that would otherwise be destroyed to help research move forward. Obtaining human skin tissue to work with doesn’t have to be a problem.
- With Genoskin’s unique technology, it is possible to maintain the functional properties of the donated skin and to keep the tissue’s natural immune cells alive. This allows studying human immune cells in their natural ecosystem to understand their crosstalk, functioning and to determine the molecular signature of the tissue.
- When the skin models are prepared, it is possible to introduce a drug, either topically, such as a cream, or via injection, just like you’d inject a live human being, to look at what happens in the tissue.
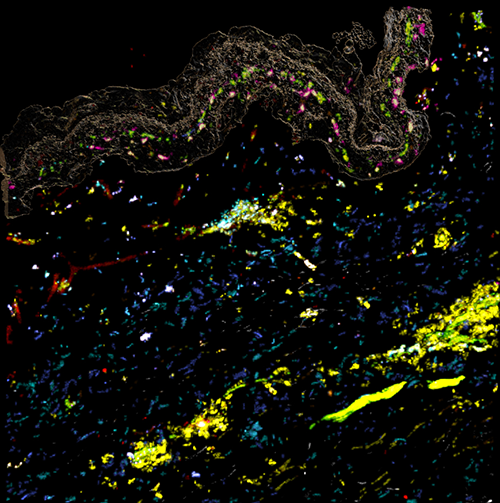
Image of simultaneous cell response in a human skin model, obtained via Genoskin’s spatial biology platform MANTIS®. Markers used: Turquoise: CD1c / Blue: CD86 / White: HLA-DR / Green: CCR7 / Yellow: CD45 / Pink: CD207 / Orange: CD80 / Red: CD40 / Light green: CD83
Ex vivo clinical trials at the preclinical stage
The real human skin tissue used in our models is immunocompetent, which is essential to characterize immune response to different doses and conditions. The skin also contains the donors’ specific characteristics and consequently, allows generating ex vivo clinical data during the preclinical trial phase, even taking population diversity into account.
Studying different populations
Clinical trials need to reflect population diversity and include different genders, ages, ethnic groups and sometimes even different medical conditions. The FDA recently stressed the importance of heterogeneity and clinical trials should include a representative sample of the final patient population. Luckily, surgeries, such as tummy-tucks, that require the removal of human skin tissue, are very common for a diverse group of individuals. Using real human skin therefore offers an excellent opportunity to start studying the diversity sought in clinical trials at the preclinical stage.

Testing different doses and conditions
Since skin is readily available through donations, this functional organ can be obtained in large amounts. This makes it possible to broaden the scope of preclinical R&D in order to compare different options and pick the best solution. It’s possible to test multiple doses in the same donor because the skin is prepared in small units. The units are about an inch in size and allow research on different conditions. With Genoskin’s skin models, a typical surgery yields roughly 50 units from one individual. This approach allows studying multiple conditions and doing replicate studies in the same individual to make sure that, from a scientific perspective, everything is sound. It therefore becomes possible to document how – sometimes – minute changes can have very large influences in the body, and adjust the development process accordingly.

Genoskin’s ex vivo human skin platforms allow for the immune profiling of drugs, vaccines and compounds that are administered via topical, systemical, intradermal and subcutaneous routes. We provide the only testing platform worldwide to test subcutaneously injected products outside of animals and humans, to not only predict immune response to drugs in the skin but also in other human organs that share a similar immune ecosystem. Our expertise includes unbiased data analysis via artificial intelligence, spatial biology and data comparison to real-world evidence and drug data for extra relevance. Don’t hesitate to get in touch for more detailed information.
Comments are closed.