Resources
8
Jul
A unique human skin model to study subcutaneous injections
HypoSkin®, an ex vivo human skin model to predict toxicity & efficacy of subcutaneous drugs Recent advancements in subcutaneous injection testing have brought about significant improvements in drug delivery and patient experience. These advancements include the development of innovative injection devices and formulations that enhance the ease and comfort of subcutaneous drug administration. Market trends also indicate a growing demandRead more
2
Jul
PROTOCOL – Subcutaneous injection
ProtocolSubcutaneous InjectionPlease fill in the form below to access the protocol. Need more info? Please contact us! Contact us
2
Jul
PROTOCOL – Intradermal injection
ProtocolIntradermal InjectionPlease fill in the form below to access our poster. After submitting this form, you will receive an email containing the link to access our poster. Need more info? Please contact us! Contact us
28
Jun
APPLICATION NOTE – HypoSkin® services
HypoSkin services combine an ex vivo skin platform with new generation transcriptomic, cytokine analysis and bioinformatics to support your R&D with human insights.
26
Jun
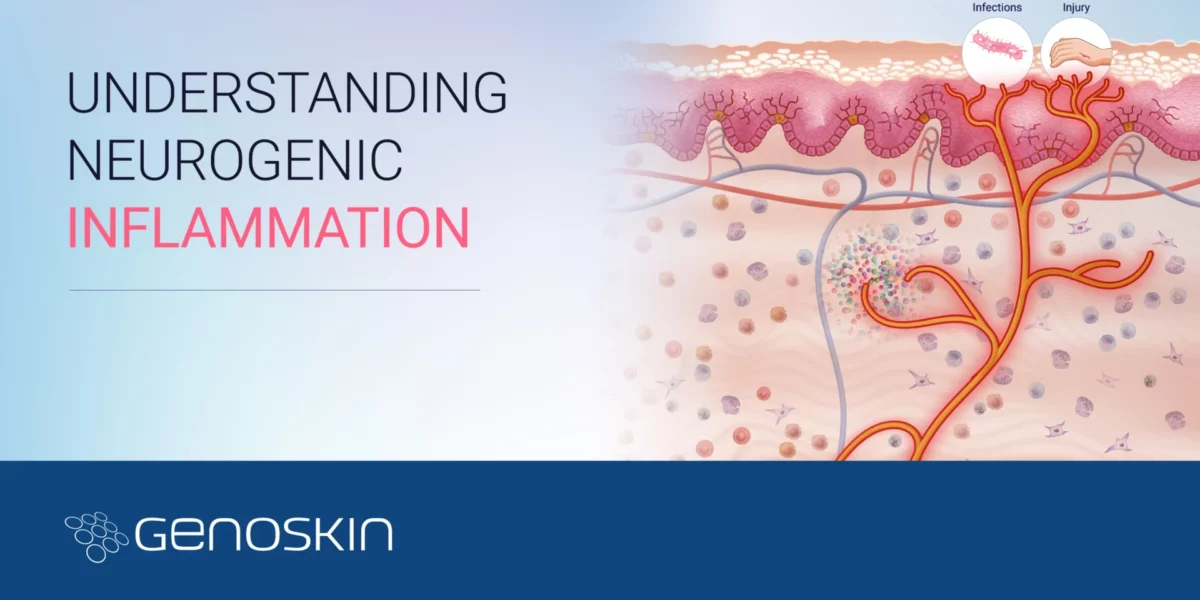
Understanding neurogenic inflammation
Understanding Pain & Neurogenic InflammationCan our understanding of pain and neurogenic inflammation help in predicting and managing injection-related pain?Nociception, the neural processes of encoding and processing noxious stimuli, and the sensation of pain are initiated by extreme pressures, temperatures, toxic substances, and inflammatory mediators capable of causing tissue injury. These strong stimuli are recognized by specific nerve cells in theRead more
26
Jun
USER MANUAL – HypoSkin®
The User Manual includes detailed descriptions of the HypoSkin® model, available formats, and a step-by-step guide for kit use.