Redefining Local Toxicity Assessment
Part 1 – The Role of Animal-Free Non-Clinical Platforms in Drug Development
In the intricate world of drug development, the quest for precision, safety, and efficacy is unending.
Traditional toxicity assessment methods using animal models, while historically significant, have shown discernible limitations in their predictability of human responses.
As the biotech and pharma sectors continue to push the boundaries of innovation, there is an imperative need to critically evaluate these traditional paradigms and transition towards more human-relevant testing platforms.
Welcome to the first article of our three-part series, where we will delve into the challenges inherent in conventional testing methods and how non-clinical platforms can revolutionize the prediction of skin toxicity upon injection.
The Limitations of Traditional Testing Methods
Inaccuracy and Variability
Despite their widespread use, traditional testing methods often grapple with the challenge of accurately replicating human responses. Animal models, for instance, have been the cornerstone of drug testing with approximately 20 million animals used for research and testing in the US as of 2020 and around 9.4 million in the EU during the same timeframe1. While their contributions to science and medicine are undeniable, the physiological differences between traditional animal models and humans can lead to potential discrepancies in results.
Animal testing, which is primarily utilized to anticipate human toxicity, frequently emerges as an unreliable predictor of drug safety in humans. Such inconsistencies not only escalate costs but also introduce delays in drug approvals, potentially sidelining drugs that could be beneficial for human use and inadvertently exposing human subjects to risks during clinical trials2.
Ethical Concerns
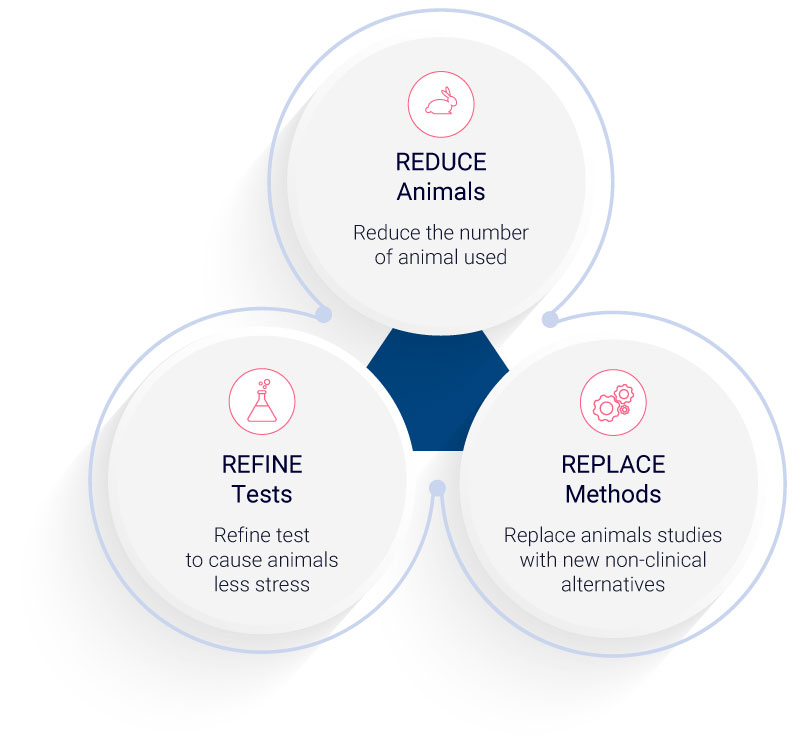
The ethical dimensions of animal testing have long been a subject of intense debate. In 1959, “The Principles of Humane Experimental Technique” introduced the world to the “3 Rs” principles: reduction, refinement, and replacement of animal use in research.3 These principles, now foundational in animal research guidelines, advocate for minimizing animal usage, alleviating their distress, and exploring non-animal alternatives. Yet, the full realization of the “3 Rs” remains elusive, and concerns linger regarding the comprehensive adoption of these principles, the evolving comprehension of animal cognition, and the genuine efficacy of animal testing in predicting human outcomes3.
Time and Cost Implications
The financial and temporal aspects of drug development are significantly impacted by the choice of testing methods. Traditional animal testing protocols can be both protracted and costly. The transition of drugs from animal testing to human treatments witnesses a staggering failure rate exceeding 92%, predominantly attributable to unforeseen toxicity or lack of efficacy4.
When a drug falters in advanced clinical trial stages due to unpredicted side effects, the financial ramifications can be profound.
In contrast, New Approach Methodologies (NAMs) like 3D cell culture models, computational modeling, and ex vivo models offer insights more attuned to human responses, showcasing greater predictability than traditional animal models.
Concluding Remark
That wraps up the first part of our series, highlighting the need for a shift from traditional toxicity testing methods, especially for those in the business of developing injectable therapeutics. These traditional methods have their place but fall short when it comes to accurately de-risking drugs designed for injection.
But there’s more to come!
In our next article, we’ll explore non-clinical alternative methods that are stepping up to meet the challenges in toxicity testing. And we’ll conclude the series with a forward-looking discussion on the future of non-clinical first-in-human studies, offering a sneak peek into the future of safer and more effective injectable drug development.
Stay tuned!
References
1A Annual number of animals used in research and testing in selected countries worldwide as of 2020. https://www.statista.com/statistics/639954/animals-used-in-research-experiments-worldwide/
2Van Norman, Gail A. “Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach?.” JACC. Basic to translational science vol. 4,7 845-854. 25 Nov. 2019
3Ferdowsian, Hope R, and Nancy Beck. “Ethical and scientific considerations regarding animal testing and research.” PloS one vol. 6,9 (2011): e24059
4Marshall LJ, Bailey J, Cassotta M, Herrmann K, Pistollato F. “Poor Translatability of Biomedical Research Using Animals — A Narrative Review“. Alternatives to Laboratory Animals. 2023;51(2):102-135.
Comments are closed.