Assessment of injection site reactions
A new proprietary research tool to identify drug-related immune adverse reactions in human skin
Today, biologics represent one of the fastest growing markets within the pharmaceutical industry.The pipeline of biological drugs is growing at a rapid pace with a global market expected to reach over 460 billion USD in 2024 (1).
In order to bypass gastric metabolism, biologics are usually designed for the parenteral route of administration. However, this approach comes with significant costs and often leads to reduced patient compliance.
In addition, subcutaneous injection offers a variety of benefits including self-medication and shorter administration time, facilitating better adhesion to the treatment and lowering overall costs. Currently, 3555 2 drug candidates are being investigated for subcutaneous routes, either new candidates or approved therapeutic products already available for parenteral routes.
However, subcutaneous injection comes with some drawbacks, including potential side effects that may significantly reduce patient compliance, thereby decreasing the effectiveness of treatments. The most reported adverse effect upon injection of a biologic drug is an injection site reaction.
While animal models remain prevalent in drug development, it is widely recognized that they may not always yield sufficient reliable data directly applicable to humans. Consequently, there is a lack of readily available models and tools that closely mimic or replicate the human immune functions and metabolism to more accurately predict such side effects.
With more than a decade of expertise in generating actionable human data using a range of live immunocompetent human skin models, Genoskin aimed to try and fill this gap. Combining an ex vivo platform with cytokine analysis and bioinformatics led to the creation of a New Approach Method centered on examining local immunotoxicity and ISR prediction.
Today, with the ImmunoSafe: ISR platform®, Genoskin provides biotechnology and pharmaceutical companies with an innovative human-based service to de-risk the development of new therapeutics. And with in vitro human primary mast cells services, we can go even deeper to identify the adverse effects mediated by these key immune cells.
What other method could offer a more accurate prediction of human responses than utilizing an actual component derived directly from a human?
But first, let’s dive a bit into injection site reaction characteristics.
Injection site reactions are triggered by a mast cell-specific receptor.
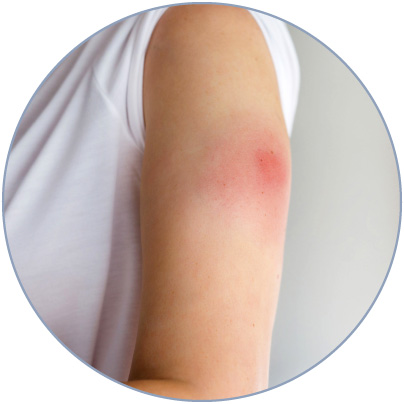
Frequently, the injection of biologics can trigger a fast and localized inflammatory response characterized by swelling, erythema, and pain.
Such a reaction is commonly known as injection site reaction (ISR), the most common reported adverse effect upon injection of a biologic drug3,4.
Immediate ISR are pseudo-allergic reactions recently reported to be triggered through a mast cells receptor, MRGPRX2, the human ortholog to the mouse MrgprB25. MRGPRX2 was found to be activated by a broad range of cationic substances such as neuropeptides, but also therapeutic drugs including beta-lactam antibiotics, chemotherapy agents, among others6.
Delayed ISRs (happening within days post-injection) can also be observed and are known to be driven by additional inflammatory mechanisms, most notably the recruitment of peripheral blood immune cells to the site of injection.
Therefore, identification of new drug-candidates that avoid eliciting mast cell-dependent or -independent inflammatory responses ahead of clinical trials is crucial to ensuring patient safety, comfort, and adherence to injectable treatment options.
MRGPRX2 was found to be activated by a broad range of cationic substances such as neuropeptides, but also therapeutic drugs including beta-lactam antibiotics, chemotherapy agents, among others6.
Delayed injection site reactions (happening within days post injection) can also be observed and are known to be driven by additional inflammatory mechanisms, most notably the recruitment of peripheral blood immune cells at the site of injection.
Therefore, identifying new drug-candidates that do not trigger mast cell dependent or independent ISR-like inflammatory responses ahead of clinical trials becomes crucial to ensure patient safety, comfort and compliance toward an injectable treatment.
How are mast cells involved in injection site reactions?
Discovered over a century ago by Paul Ehrlich, mast cells are becoming a hot topic with more than 220,000 publications over the past 6 years8.
They play a central role in allergic reactions and are indispensable for the body’s defense against pathogens. Hence, they are an asset in developing new drugs or improving existing on-market therapeutics.
To date, research on mast cells can be performed using rodent or human cell lines as well as human primary mast cells derived from peripheral blood (PB) or cord blood (CB). Mast cells can also be studied in situ, directly in living immunocompetent skin models.
Mast cells express various activatory receptors (some of them are depicted in Figure 1), and scientists are advancing their understanding of the metabolism and molecular mechanisms behind their biological functions.
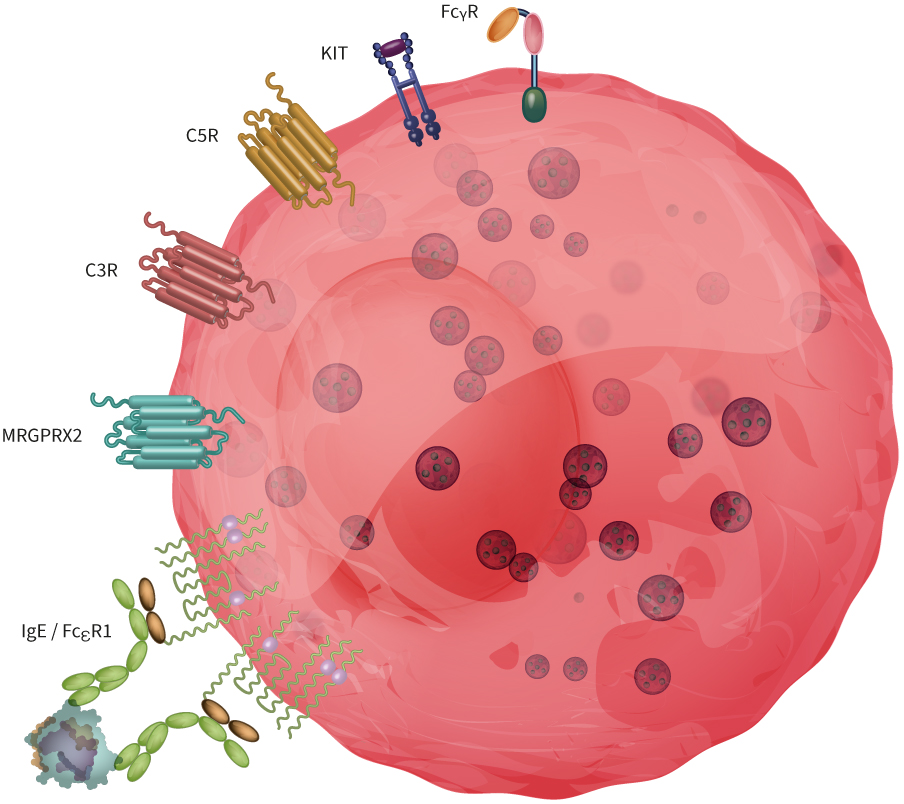
Figure 1: Mast cells express a broad range of receptors involved in acute allergic reactions and defense mechanisms against pathogens. Here, we illustrated FcεRI, MRGPRX2, C2R and C5R, KIT and FcRIIa.
Below, we describe two key mast cell receptors involved in allergic and pseudoallergic reactions in more detail.
The high affinity IgE receptor (FceRI).
Mast cells are known for their involvement in IgE-mediated disorders through their expression of the FcRI receptor.
Under resting conditions, IgE antibodies bind to FcεRI, forming a complex that diffuses freely within the cell membrane. Upon binding of an antigen to this surface-bound IgE with the correct specificity, there is a rearrangement and crosslinking of the IgE-FcεRI complexes, which subsequently activates the mast cell, leading to degranulation. This will then trigger the release of pre-stored pro-inflammatory mediators9 such as histamine and proteases, and the secretion of newly synthesized mediators such as cytokines or eicosanoids10.
MRGPRX2
As mentioned earlier, the Human Mass-related G protein-coupled receptor X2 (MRGPRX2) is highly expressed in connective tissue-type mast cells mainly located in the human skin. MRGPRX2 has been recently described as a major player in immediate injection site reaction, a severe hypersensitivity reaction that occurs in a significant number of patients treated with biologics.
IgE-dependent or MRGPRX2-dependent mast cell activation typically results in an increase of vascular permeability, formation of oedema which leads to various symptoms depending on the location of the mast cells. In the skin, common symptoms include urticarial reactions characterized by itchiness and pain.
Injection site reaction assessment: Two approaches for a deep understanding of drug-induced immune-related adverse events
Genoskin’s solution to the challenges associated with studying ISR is based on two platforms which aim to support safer drug candidate development.
At the organ level, HypoSkin® represents an advanced ex vivo human skin model that is both immunocompetent and suitable for subcutaneous injections. This model is an integral part of the ImmunoSafe: ISR Platform®, which facilitates the identification of inflammatory signatures of therapeutic compounds and predicts potential toxic immune reactions. Routine assays can include multiplex cytokine analysis, while more in-depth insights can be achieved through transcriptomic analyses. Our robust sourcing capabilities allow for the examination of compounds across diverse cohorts, varying in gender, age, and ethnicity, thereby enhancing the predictability of clinical data prior to clinical trials.
At the cellular level, our unique in vitro platform utilizes in vitro-differentiated human primary mast cells, specifically connective tissue-type mast cells, offering unprecedented insights into the molecular mechanisms underlying off-target immune reactions. When exposed to pharmaceutical compounds in the culture media, these mast cells can become activated and undergo degranulation. Alternatively, they can be pre-activated to evaluate the efficacy of therapeutics designed to prevent or interrupt mast cell activation.
These platforms can function independently or be integrated. This coupling enables a comprehensive exploration of your therapeutic mechanism of action and its safety profile at the tissue level and cellular level, providing a holistic assessment of its potential impact.
Sources
1. Globenewswire.com
2. Who.int
3. The-dermatologist.com
4. Businessinsider.com
5. McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015 Mar 12;519(7542):237-41. doi: 10.1038/nature14022. Epub 2014 Dec 17. PMID: 25517090; PMCID: PMC4359082.
6. Porebski G, Kwiecien K, Pawica M, Kwitniewski M. Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) in Drug Hypersensitivity Reactions. Front Immunol. 2018 Dec 20;9:3027. doi: 10.3389/fimmu.2018.03027. PMID: 30619367; PMCID: PMC6306423.
7. Wang et al. “The return of the mast cell: New roles in neuroimmune Itch biology”JID 140-5 P945-951, 2020.
8. App.dimensions.ai
9. Krystel-Whittemore, Melissa, et al. “Mast Cell: A Multi-Functional Master Cell.” 2016, https://www.frontiersin.org/articles/10.3389/fimmu.2015.00620/full#h1.
10. Amin K. The role of mast cells in allergic inflammation. Respir Med. 2012 Jan;106(1):9-14. doi: 10.1016/j.rmed.2011.09.007. Epub 2011 Nov 22. PMID: 22112783.
Comments are closed.