Blog
10
Jan
Immunogenicity series: Defining immunogenicity
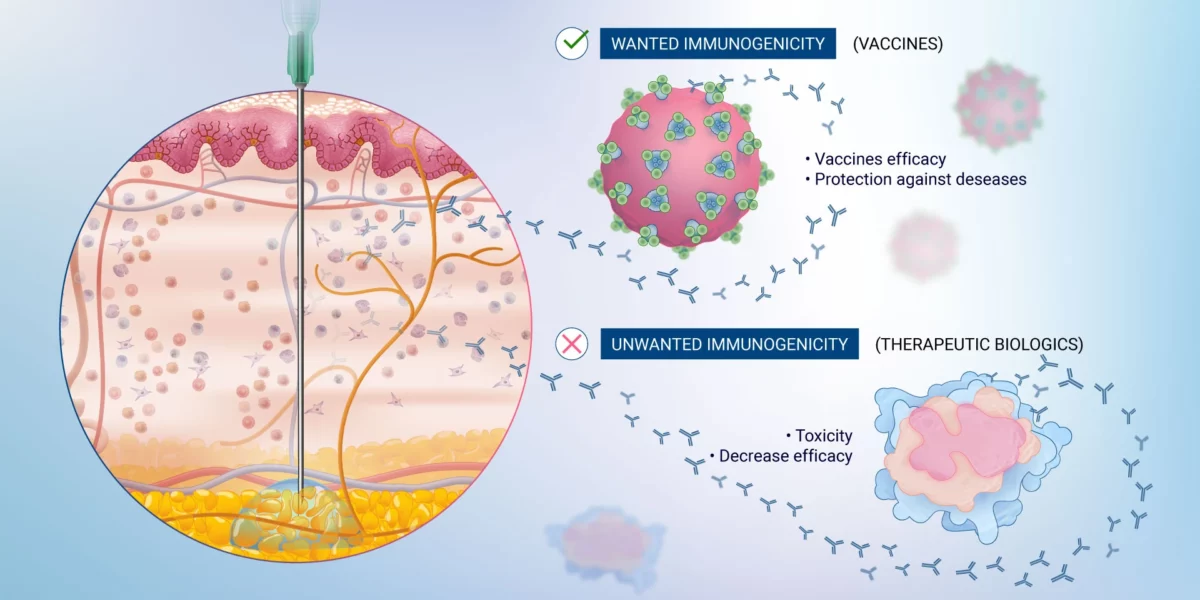
Immunogenicity SeriesPart 1 - Defining immunogenicity Immunogenicity plays a pivotal role in drug and vaccine development and is essential for optimizing therapeutic efficacy and safetyby understanding the immune system's response to the compounds being tested. This is particularly crucial in the era of biologics and personalized medicine, where the fine balance between therapeutic benefit and immune reactivity is critical. WhetherRead more
31
Oct
The role of animal-free non-clinical platforms in drug development – Part 2
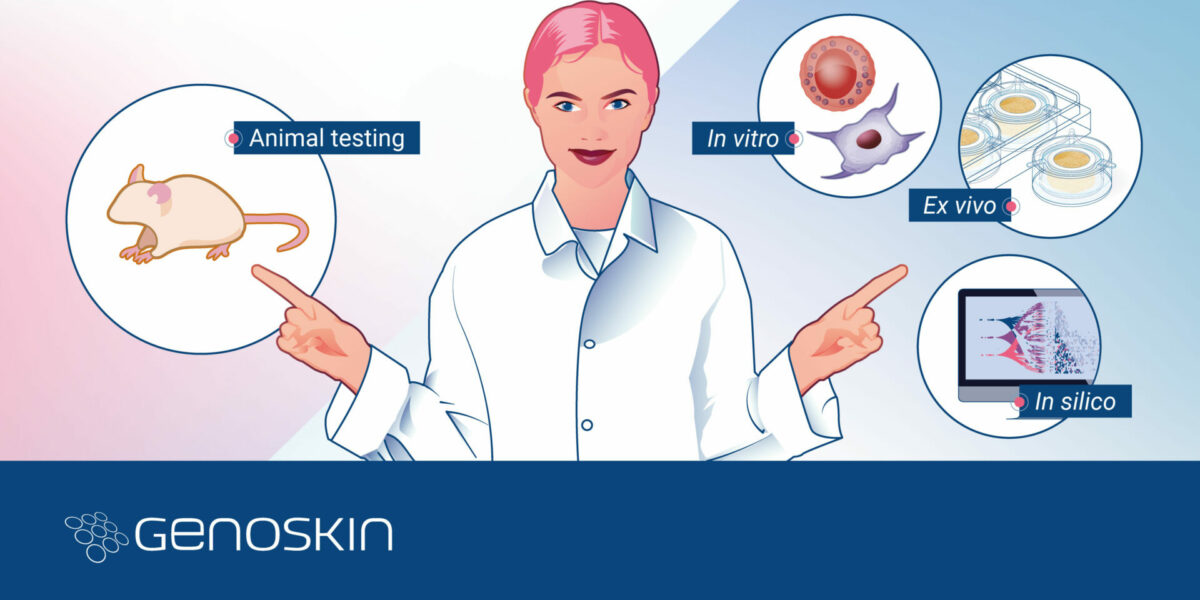
Redefining Local Toxicity AssessmentPart 2 - The Role of Animal-Free Non-Clinical Platforms in Drug Development. In the realm of non-clinical animal-free platforms, a transformative shift is unfolding in response to the challenges posed by drug-induced adverse reactionsa predominant factor leading to the discontinuation of drug development projects and the withdrawal of drugs from the market1. The imperative for accurate, ethical,Read more
6
Oct
The role of animal-free non-clinical platforms in drug development – Part 1
Redefining Local Toxicity AssessmentPart 1 - The Role of Animal-Free Non-Clinical Platforms in Drug DevelopmentIn the intricate world of drug development, the quest for precision, safety, and efficacy is unending.Traditional toxicity assessment methods using animal models, while historically significant, have shown discernible limitations in their predictability of human responses. As the biotech and pharma sectors continue to push the boundariesRead more
25
Apr
Why testing and mitigating Injection Site Reactions is critical during drugs/vaccines development ?
Why testing & mitigating Injection Site Reactions is critical during drugs/vaccines development? Injection site reactions (ISR) are a common occurrence when a person receives an injection in the skin, such as a vaccine or self-administered biologics.These reactions occur due to the activation of the immune system at the injection site. When a foreign substance, such as a vaccine or medication,Read more
31
Aug
Human skin to predict clinical outcomes
Human skin to better predict clinical trial outcomes Based on the Tech Nation interview with Dr. Eric Merle, our Chief Bussiness Innovation Officer by Moira Gunn, in February 2022. The entire Tech Nation Radio Podcast is available here. Eric's interview starts at 44:00 mins. Close is not good enough We've grown used to testing drugs in animals because some animalsRead more
31
May
Immunocompetent human skin for vaccine and drug development
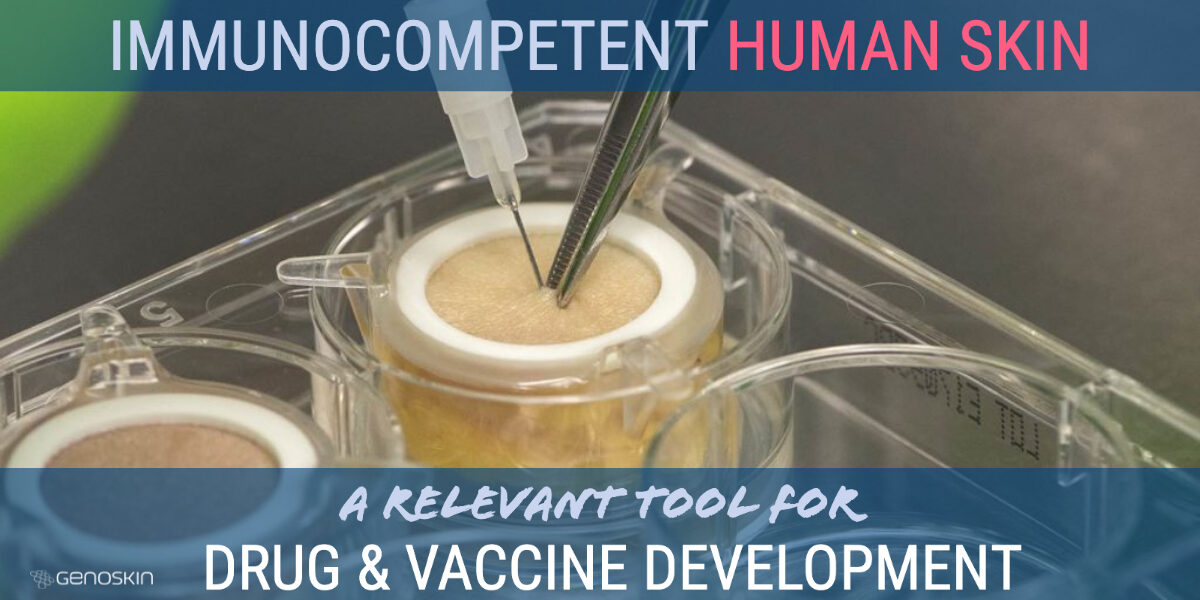
Before proceeding to clinical trials in humans, today’s scientists study drug efficacy and toxicity in animals. Even though animal studies clearly raise ethical questions, it is considered even more unethical to conduct studies directly on humans without prior testing. Genoskin provides a unique alternative to animal testing and to human trials, using real, immunocompetent human skin. Why human skin isRead more