Drug Development
4
Mar
WEBINAR #10 – Subcutaneous administration of vesicant/irritant anticancer drugs from water-soluble polymer prodrugs
Top-level science through the eyes of an expert.Join us for the next session in Genoskin’s webinar series, where we explore cutting-edge advancements in drug delivery and therapeutic innovation. This session will feature an in-depth presentation on polymer prodrug nanocarriers, showcasing a novel approach to controlled and efficient drug release. Watch the replayThis webinar explores new research on polymer-drug conjugates inRead more
10
Feb
From human skin to human data: Advancing immunogenicity testing with human models
From human skin to human data: Advancing immunogenicity testing with human modelsIn a public workshop organized by the FDA-CRCG group, Dr. Nicolas Gaudenzio, CSO of Genoskin, introduced an innovative platform for assessing immune reactions in response to injected substances using human skin explants. His talk, titled "A new in vitro framework for assessing immunogenicity using comprehensive profiling of injectable humanRead more
14
Oct
POSTER – NativeSkin®, an immunocompetent skin model to study antigen uptake
Skin ModelsNativeSkin®, an immunocompetent human skin model to study antigen uptake and processing by Langerhans cells Research by E. Pagès1, L. Mondoulet3, E. Bonnefont1, E. Braun1, V. Dhelft2, C. Dupont3, H. Sampson2, P. Descargues1 1Genoskin, 2DBV Technologies, 3Hôpital Necker, Université Paris-Descartes Scientific Context Epicutaneous immunotherapy (EPIT) using Viaskin® patches has been shown to induce tolerance in sensitized mice (Dioszeghy, ClinRead more
20
Sep
WEBINAR #8 – VaxSkin – Manon Scholaert
Top-level science through the eyes of an expert.The eighth Webinar. This webinar episode is open to English-speaking participants with a solid scientific background. Featured speaker is Dr. Manon Scholaert, Scientist at Genoskin. Watch the replayIn this webinar, Dr. Manon Scholaert will walk us through her cutting-edge research that utilizes advanced bioengineering techniques to study the interactions of immune cells inRead more
22
Jul
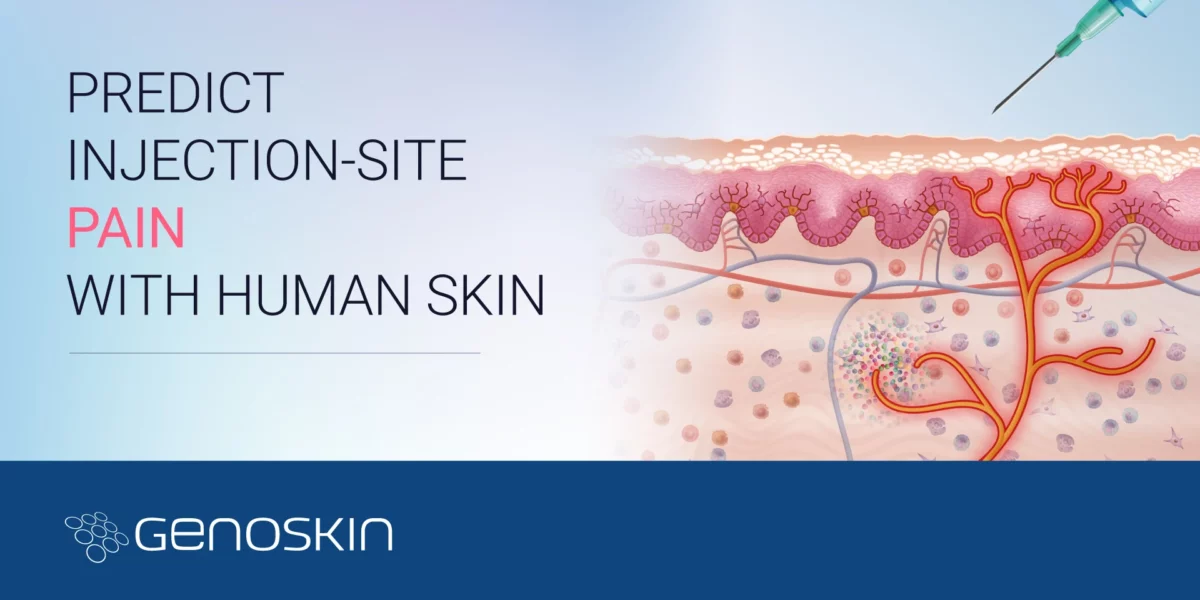
Injection-Site Pain
Injection-Site PainWhich factors influence pain upon subcutaneous injection?Biologics such as vaccines, insulin, monoclonal antibodies, etc., that are now widely developed need to be administered parenterally. While the intravenous (IV) route has historically been the most widely used, the subcutaneous (SC) route is now accepted and presented as safe and effective, whether in the context of chronic disease or in palliativeRead more
16
May
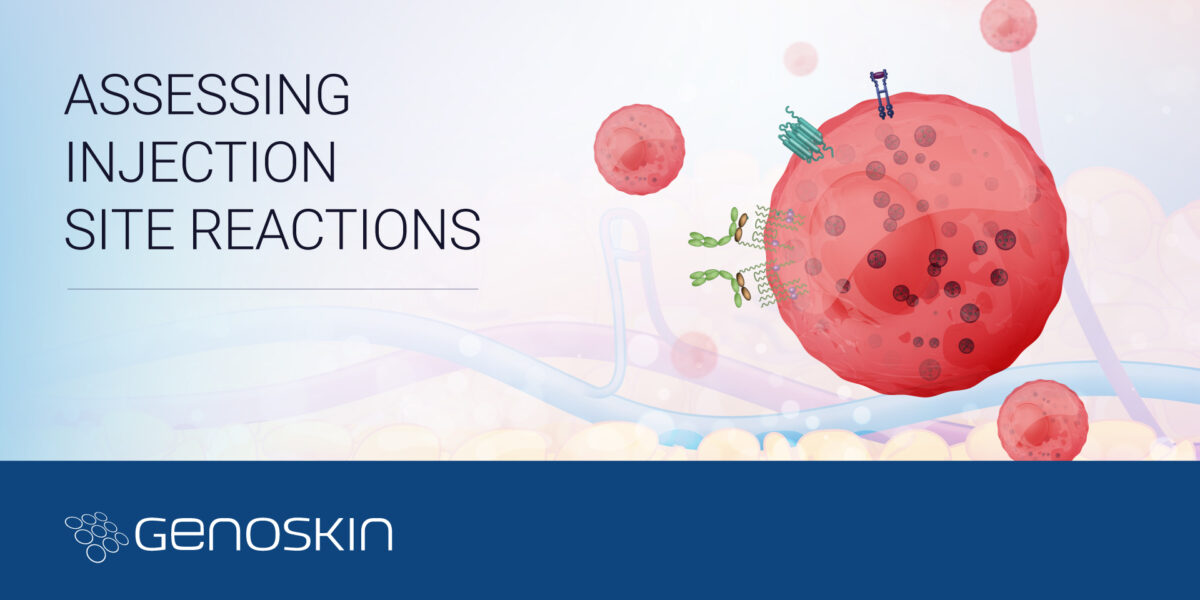
Injection site reactions platform
Assessment of injection site reactionsA new proprietary research tool to identify drug-related immune adverse reactions in human skinToday, biologics represent one of the fastest growing markets within the pharmaceutical industry.The pipeline of biological drugs is growing at a rapid pace with a global market expected to reach over 460 billion USD in 2024 (1). In order to bypass gastric metabolism,Read more