Blog
16
Apr
Investigative toxicology
Transforming Drug SafetyThe Revolutionary Role of Investigative Toxicology in Drug DevelopmentIn an era where the pace of pharmaceutical innovation is rapidly evolving, the field of toxicology is undergoing a transformative shift. This goes beyond subtle changes; there is a fundamental reimagining of its role from observing drug-induced effects to proactively shaping drug safety measures.The Integration of Investigative Toxicology The traditionalRead more
18
Mar
Genoskin at the World Vaccine Congress in DC!
Genoskin at the World Vaccine CongressAdvancing Vaccine Research with High-Dimensional Immune ProfilingFrom April 1-4, 2024, the global vaccine community gathered for the World Vaccine Congress in Washington, D.C. Taking place at the heart of the nation's capital, the congress was a cornerstone event for vaccine professionals worldwide, offering a platform for sharing groundbreaking research, fostering collaborations, and exploring the latestRead more
29
Feb
The silent crisis in biomedical research: beyond macaques
The Silent Crisis in Biomedical Research: Beyond MacaquesFinding an alternative to non-human primates to evaluate human therapeutics In a compelling narrative echoed by Le Monde, the biomedical sector faces a silent crisis, highlighting the geopolitical intricacies and ethical quandaries of scientific research. At its core, the dwindling supply of non-human primates (NHPs), particularly macaques, essential for testing medical treatments, hasRead more
29
Feb
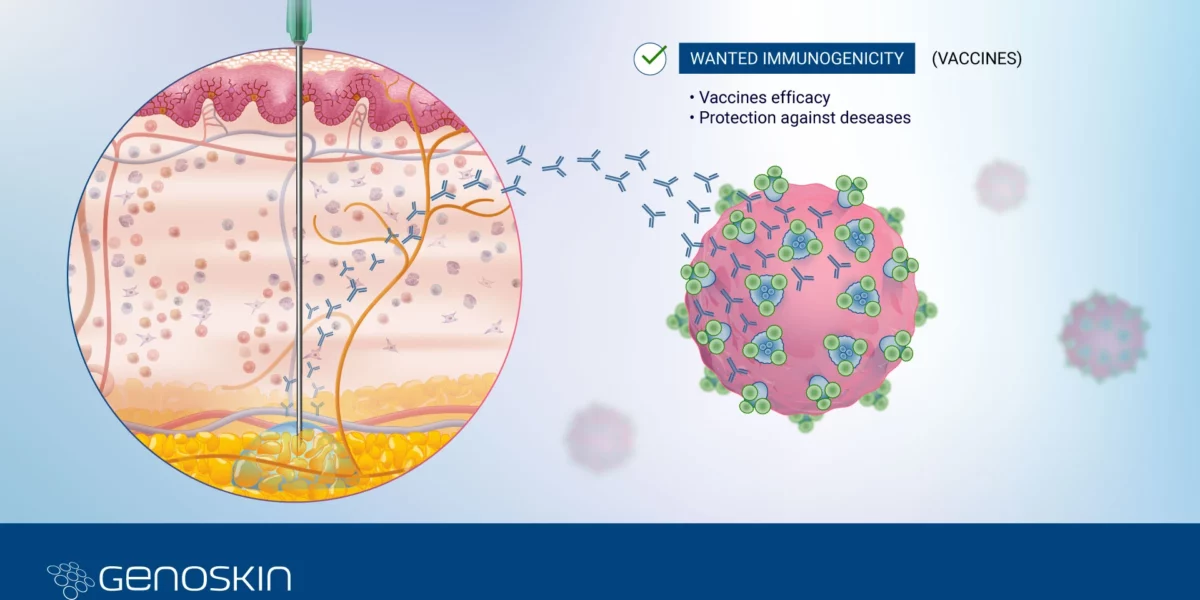
Immunogenicity series: Wanted immunogenicity for vaccine development
Immunogenicity SeriesPart 2 - Wanted Immunogenicity for Vaccine DevelopmentImmunogenicity is a key concept in vaccine development,referring to the ability of a vaccine to stimulate an immune response in the recipient. It is a desirable and critical characteristic because the effectiveness of a vaccine is directly linked to its ability to induce a robust and protective immune response. Vaccine Development: GlobalRead more
15
Feb
Genoskin at SOT 63rd Annual Meeting and ToxExpo
Genoskin at SOT 63rd Annual Meeting and ToxExpoPioneering Human Skin Models for Advanced Toxicology ResearchAs the world of toxicology convened for the Society of Toxicology 63rd Annual Meeting and ToxExpo, Genoskin was excited to announce its participation in this event, which took place from March 10-14 at the Salt Palace Convention Center in Salt Lake City. This year, Genoskin showcasedRead more
9
Feb
Understanding local tolerance
Understanding Local Tolerance in drug developmentIn the ever-evolving field of drug development, ensuring the safety and efficacy of new therapeutic agents is paramount. A crucial aspect of this safety evaluation is the assessment of cytotoxicity – the potential of substances to harm or destroy cells. Particularly in the realm of skin cytotoxicity, the concept of Local Tolerance, especially in theRead more