Blog
8
Jul
A unique human skin model to study subcutaneous injections
HypoSkin®, an ex vivo human skin model to predict toxicity & efficacy of subcutaneous drugs Recent advancements in subcutaneous injection testing have brought about significant improvements in drug delivery and patient experience. These advancements include the development of innovative injection devices and formulations that enhance the ease and comfort of subcutaneous drug administration. Market trends also indicate a growing demandRead more
26
Jun
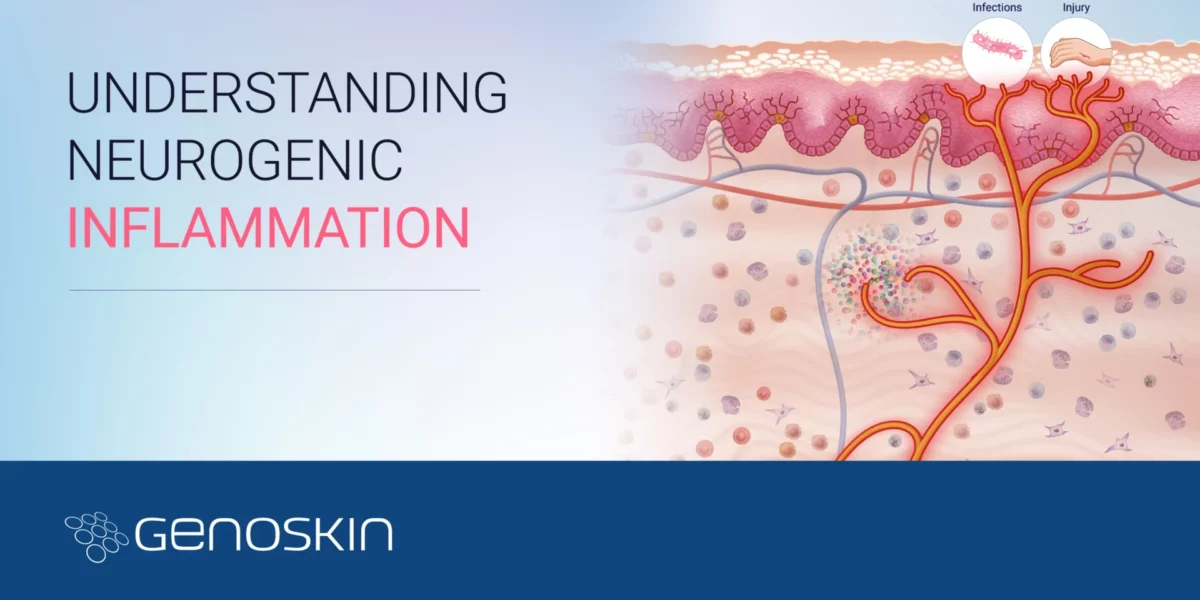
Understanding neurogenic inflammation
Understanding Pain & Neurogenic InflammationCan our understanding of pain and neurogenic inflammation help in predicting and managing injection-related pain?Nociception, the neural processes of encoding and processing noxious stimuli, and the sensation of pain are initiated by extreme pressures, temperatures, toxic substances, and inflammatory mediators capable of causing tissue injury. These strong stimuli are recognized by specific nerve cells in theRead more
21
May
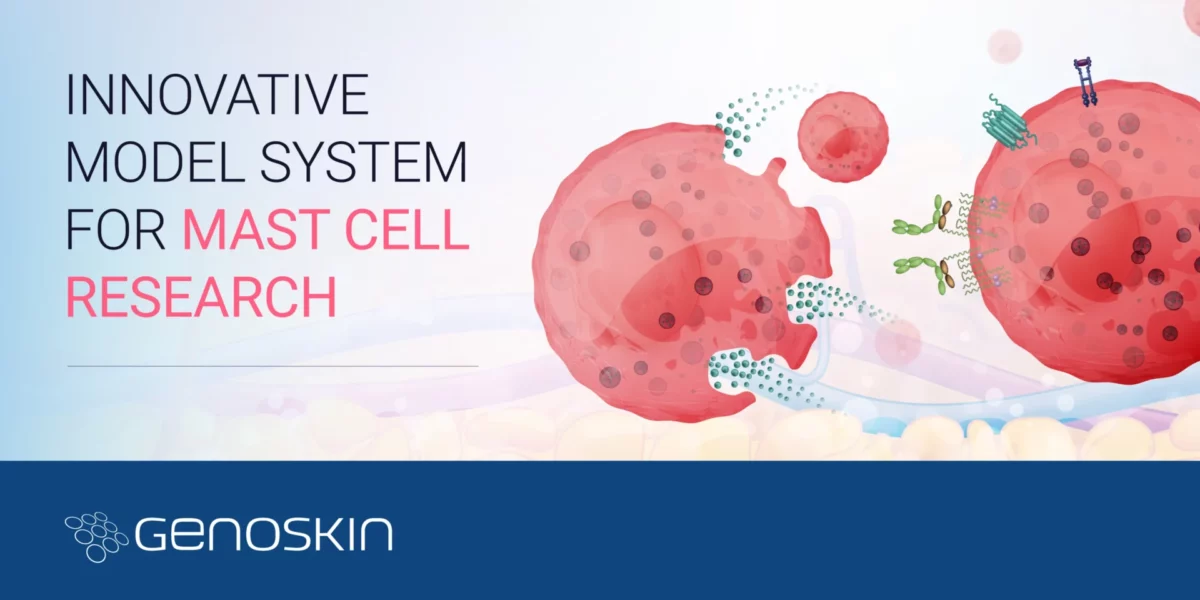
Ask the Expert: Your top questions about Genoskin’s mast cell platform answered
Ask the Expert: Your Top Questions About Genoskin’s Mast Cell Platform Answered Curious about mast cells and their role in injection site reactions? Learn about how Genoskin sources and cultures human primary mast cells, the unique qualities of our mast cell research solution, and its diverse applications in drug toxicity assessment, allergic reaction evaluation, and vaccine development. 1. How doRead more
16
May
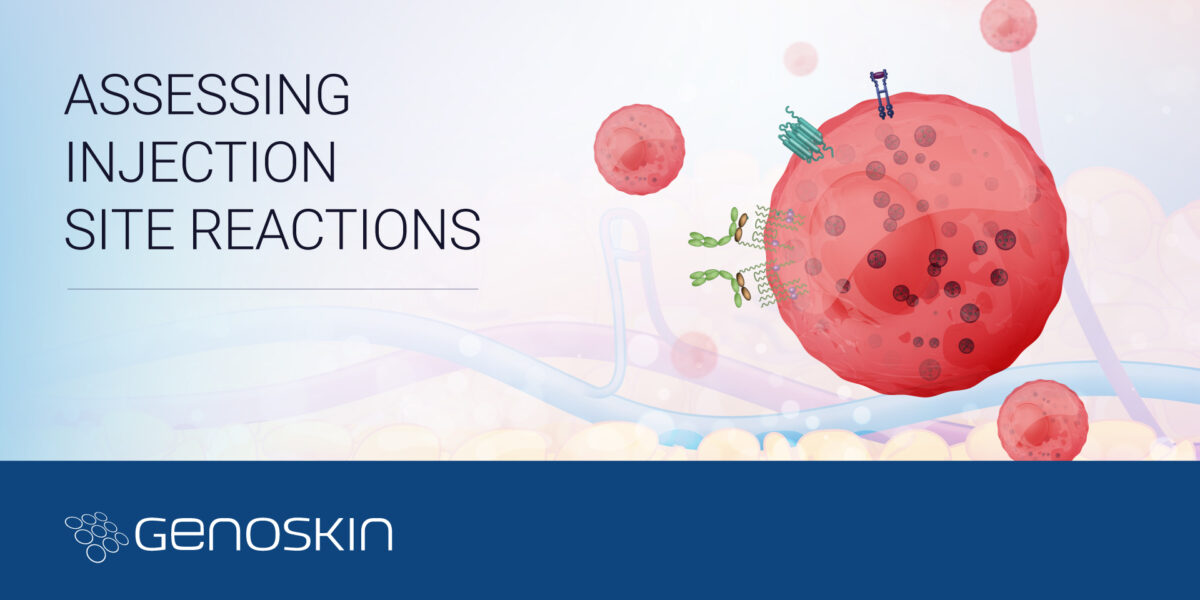
Injection site reactions platform
Assessment of injection site reactionsA new proprietary research tool to identify drug-related immune adverse reactions in human skinToday, biologics represent one of the fastest growing markets within the pharmaceutical industry.The pipeline of biological drugs is growing at a rapid pace with a global market expected to reach over 460 billion USD in 2024 (1). In order to bypass gastric metabolism,Read more
2
May
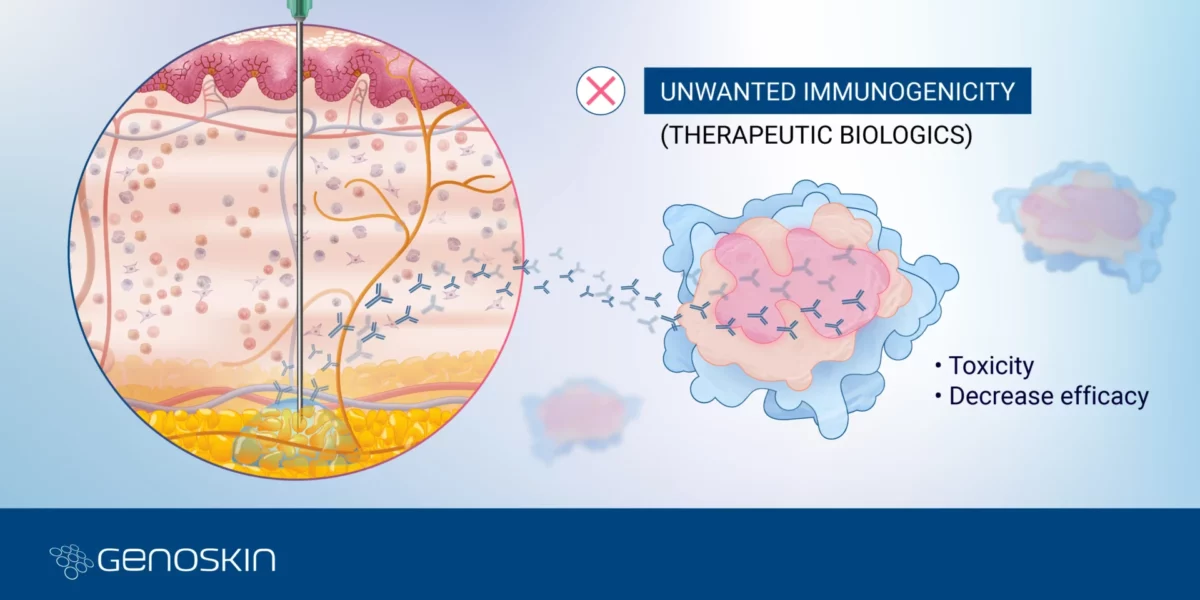
Unwanted immunogenicity
Navigating Unwanted ImmunogenicityPart 3 - Challenges & insights in biologic drug developmentOver the past two and a half decades, biologics development has been growing exponentially, matching the pace of small molecules development in 20221. Biologics, or biopharmaceutical products, encompass a wide range of therapeutic products such as vaccines, monoclonal antibodies, gene and cell therapy, and recombinant and fusion therapeutic proteins.Read more
17
Apr
Genoskin at the European Mast Cell and Basophil Research Network meeting
Genoskin at the European Mast Cell and Basophil Research Network meeting in ToulouseAdvancing mast cell research through in vitro evaluation of mast cell responsesWe were thrilled to participate in the prestigious European Mast cell and Basophil Research Network (EMBRN) Conference, which took place in Genoskin’s hometown, Toulouse, France, from May 29-31, 2024. This event brought together prominent academic researchers, clinicians,Read more