Toxicity Assessment
16
May
Injection site reactions platform
Assessment of injection site reactionsA new proprietary research tool to identify drug-related immune adverse reactions in human skinToday, biologics represent one of the fastest growing markets within the pharmaceutical industry.The pipeline of biological drugs is growing at a rapid pace with a global market expected to reach over 460 billion USD in 2024 (1). In order to bypass gastric metabolism,Read more
2
May
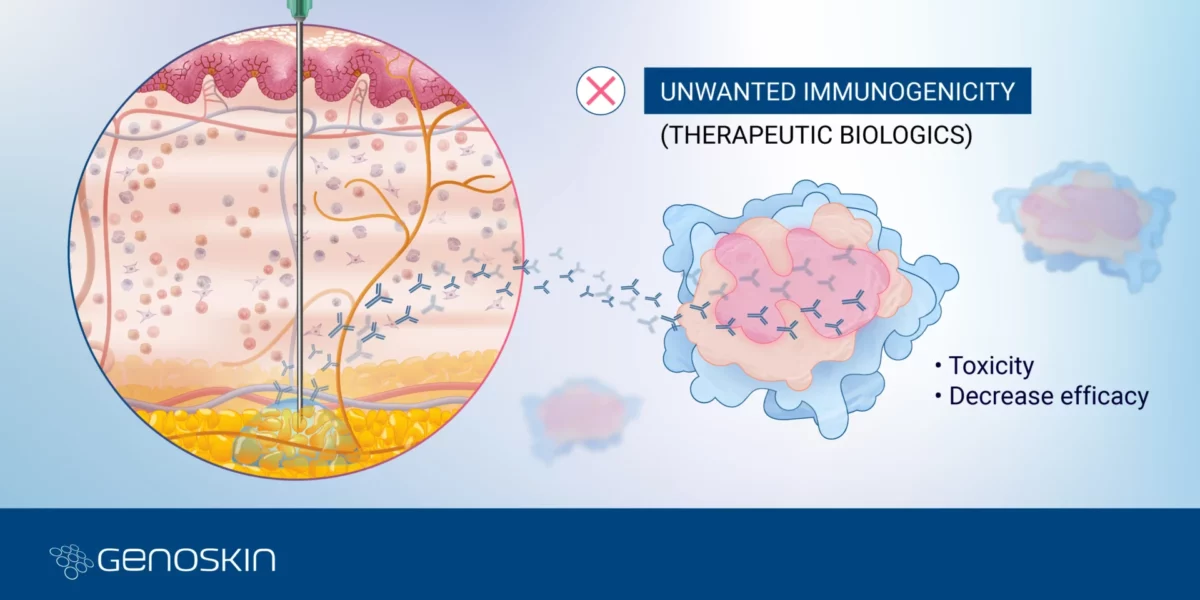
Unwanted Immunogenicity
Navigating Unwanted ImmunogenicityPart 3 - Challenges & insights in biologic drug developmentOver the past two and a half decades, biologics development has been growing exponentially, matching the pace of small molecules development in 20221. Biologics, or biopharmaceutical products, encompass a wide range of therapeutic products such as vaccines, monoclonal antibodies, gene and cell therapy, and recombinant and fusion therapeutic proteins.Read more
16
Apr
Investigative toxicology
Transforming Drug SafetyThe Revolutionary Role of Investigative Toxicology in Drug DevelopmentIn an era where the pace of pharmaceutical innovation is rapidly evolving, the field of toxicology is undergoing a transformative shift. This goes beyond subtle changes; there is a fundamental reimagining of its role from observing drug-induced effects to proactively shaping drug safety measures.The Integration of Investigative Toxicology The traditionalRead more
9
Feb
Understanding Local Tolerance
Understanding Local Tolerancein Drug DevelopmentIn the ever-evolving field of drug development, ensuring the safety and efficacy of new therapeutic agents is paramount. A crucial aspect of this safety evaluation is the assessment of cytotoxicity – the potential of substances to harm or destroy cells. Particularly in the realm of skin cytotoxicity, the concept of Local Tolerance, especially in the contextRead more
31
Oct
The Role of Animal-Free Non-Clinical Platforms in Drug Development – Part 2
Redefining Local Toxicity AssessmentPart 2 - The Role of Animal-Free Non-Clinical Platforms in Drug Development. In the realm of non-clinical animal-free platforms, a transformative shift is unfolding in response to the challenges posed by drug-induced adverse reactionsa predominant factor leading to the discontinuation of drug development projects and the withdrawal of drugs from the market1. The imperative for accurate, ethical,Read more
6
Oct
The Role of Animal-Free Non-Clinical Platforms in Drug Development – Part 1
Redefining Local Toxicity AssessmentPart 1 - The Role of Animal-Free Non-Clinical Platforms in Drug DevelopmentIn the intricate world of drug development, the quest for precision, safety, and efficacy is unending.Traditional toxicity assessment methods using animal models, while historically significant, have shown discernible limitations in their predictability of human responses. As the biotech and pharma sectors continue to push the boundariesRead more